Isometheptene, Caffeine, and Acetaminophen Tablets Prescribing Information
Package insert / product label
Dosage form: tablet
Drug class: Analgesic combinations
Medically reviewed by Drugs.com. Last updated on Jun 15, 2023.
On This Page
WARNING
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often in combination with other acetaminophen-containing products.
Isometheptene, Caffeine, and Acetaminophen Tablets Description
Each Isometheptene Mucate, Caffeine, and Acetaminophen Caplet contains:
Isometheptene Mucate ..... 65 mg
Caffeine ..... 20 mg
Acetaminophen ..... 325 mg
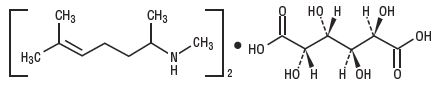
Isometheptene Mucate is a white crystalline powder having a characteristic aromatic odor and bitter taste. It is an unsaturated aliphatic amine with sympathomimetic properties. Isometheptene mucate has the following structural formula:
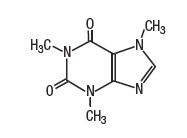
Caffeine is 1H-Purine-2,6-dione, 3,7-dyhydro-1,3,7-trimethyl-. It has the following structural formula:
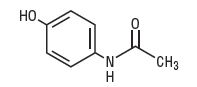
Acetaminophen, a non-salicylate, occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste. Acetaminophen is Acetamide, N-(4-hydroxyphenyl)-. It has the following structural formula:
Inactive ingredients include colloidal silicon dioxide, crospovidone polyplasdone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, and stearic acid.
Isometheptene, Caffeine, and Acetaminophen Tablets - Clinical Pharmacology
Isometheptene mucate, a sympathomimetic amine, acts by constricting dilated cranial and cerebral arterioles, thus reducing the stimuli that lead to vascular headaches. It is particularly desirable in patients predisposed to nausea and vomiting, and where ergotamines are precluded. Its action is similar to ergotamine but possesses a low order of toxicity.
Caffeine, also a cranial vasoconstrictor, is added to further enhance the vasoconstrictor effect. It is also used as a central stimulant for relief of headache.
Acetaminophen, an effective non-narcotic analgesic, reduces the perception of pain impulses originating from dilated cerebral vessels; no hyperacidity of stomach and less allergies than aspirin.
Indications and Usage for Isometheptene, Caffeine, and Acetaminophen Tablets
For relief of tension and vascular headaches.*
*Based on a review of this drug (isometheptene mucate), the National Academy of Sciences- National Research Council and/or other information, FDA has classified the other indication as “Possibly” effective in the treatment of migraine headache. Final classification of less than effective indication requires further investigation.
Contraindications
Isometheptene Mucate, Caffeine, and Acetaminophen is contraindicated in Glaucoma and/or severe cases of renal disease, hypertension, organic heart disease, hepatic disease, and in those patients who are on monoamine oxidase inhibitor (MAOI) therapy.
Warnings
Hepatotoxicity - Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products. The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4,000 milligrams of acetaminophen per day, even if they feel well.
Serious Skin Reactions - Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Patients should be informed about the signs of severe skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Hypersensitivity/Anaphylaxis – There have been post-marketing reports of hypersensitivity and anaphylaxis associated with the use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue Isometheptene Mucate, Caffeine, and Acetaminophen Caplets immediately and seek medical care if they experience these symptoms. Do not prescribe Isometheptene Mucate, Caffeine, and Acetaminophen Caplets for patients with acetaminophen allergy.
Precautions
Caution should be observed in hypertension, peripheral vascular disease and after recent cardiovascular attacks.
Information for Patients
Do not take this product if you are allergic to any of its ingredients. If you develop signs of allergy such as rash or difficulty breathing stop taking this product and contact your healthcare provider immediately.
Do not take more than 4,000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Adverse Reactions/Side Effects
Hypersensitive patients have shown rash and transient dizziness, this can be eliminated by reducing dosage.
Overdosage
Following an acute overdosage, toxicity may result.
Acetaminophen – In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Isometheptene, Caffeine, and Acetaminophen Tablets Dosage and Administration
FOR RELIEF OF MIGRAINE HEADACHE: The usual adult dosage is one or two caplets at once, followed by one caplet every hour until relieved, up to 5 caplets within a twelve hour period.
FOR RELIEF OF TENSION HEADACHE: The usual adult dosage is one or two caplets every four hours up to 8 caplets a day.
How is Isometheptene, Caffeine, and Acetaminophen Tablets supplied
Isometheptene Mucate, Caffeine, and Acetaminophen Caplets are supplied as white caplets debossed “SP 325” on one side. The opposite side is plain. Available in bottles of 50 caplets, NDC 42195-145-50.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, CALL A DOCTOR OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
| ISOMETHEPTENE MUCATE, CAFFEINE, AND ACETAMINOPHEN
isometheptene mucate, caffeine and acetaminophen tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Xspire Pharma (078312042) |
More about acetaminophen / caffeine / isometheptene mucate
- Check interactions
- Compare alternatives
- Reviews (9)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: analgesic combinations


