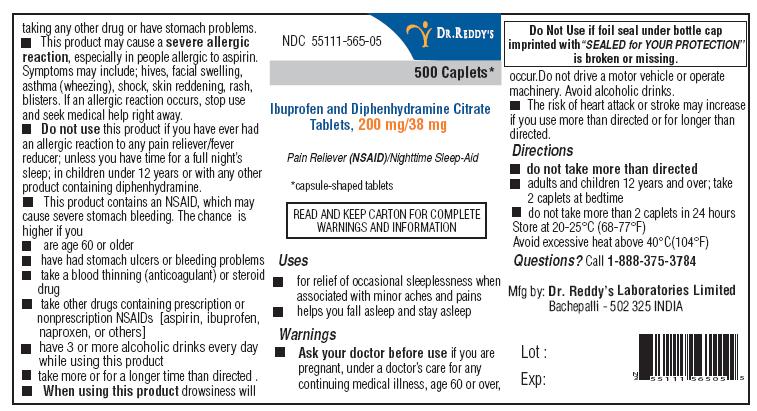
Ibuprofen and Diphenhydramine Prescribing Information
Package insert / product label
Generic name: ibuprofen and diphenhydramine citrate
Dosage form: tablet
Drug class: Analgesic combinations
Medically reviewed by Drugs.com. Last updated on Feb 28, 2024.
On This Page
ACTIVE INGREDIENTS (IN EACH CAPLET)
Diphenhydramine citrate USP, 38 mg
Ibuprofen USP, 200 mg (NSAID)**
* capsule-shaped tablets
**nonsteroidal anti-inflammatory drug
Indications and Usage for Ibuprofen and Diphenhydramine
- for relief of occasional sleeplessness when associated with minor aches and pains
- helps you fall asleep and stay asleep
Warnings
Allergy alert:
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning:
This product contains an NSAID, which may cause stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- unless you have time for a full night's sleep
- in children under 12 years of age
- right before or after heart surgery
- with any other product containing diphenhydramine, even one used on skin
- if you have sleeplessness without pain.
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, or asthma
- you are taking a diuretic
- you have a breathing problem such as emphysema or chronic bronchitis
- you have glaucoma
- you have trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers, or any other sleep-aid
- under a doctor’s care for any continuing medical illness
- taking any other antihistamines
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- redness or swelling is present in the painful area
- any new symptoms appear
Ibuprofen and Diphenhydramine Dosage and Administration
- do not take more than directed
- adults and children 12 years and over: take 2 caplets at bedtime.
- do not take more than 2 caplets in 24 hours
OTHER INFORMATION
- read all warnings and directions before use. Keep carton.
- store at 20-25°C (68-77°F)
- avoid excessive heat above 40°C (104°F)
INACTIVE INGREDIENTS
carnauba wax, corn starch, colloidal silicon dioxide, croscarmellose sodium, FD&C blue no. 2, hypromelllose, microcrystalline cellulose, polydextrose, polyethylene glycol 400, pregelatinized starch, sodium lauryl sulfate, stearic acid, titanium dioxide
| IBUPROFEN AND DIPHENHYDRAMINE CITRATE
ibuprofen and diphenhydramine citrate tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Dr. Reddy's Laboratories Limited (650562841) |
More about diphenhydramine / ibuprofen
- Check interactions
- Compare alternatives
- Reviews (21)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: analgesic combinations
- En español
Patient resources
Professional resources
Other brands
Advil PM, Ibuprofen PM, Motrin PM