Hepzato Prescribing Information
Package insert / product label
Generic name: melphalan hydrochloride
Dosage form: injection, powder, lyophilized, for solution
Drug class: Alkylating agents
Medically reviewed by Drugs.com. Last updated on Dec 14, 2023.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
HEPZATO (melphalan) for injection is a component of the HEPZATO KIT Hepatic Delivery System (HDS) for intra-arterial use.
Initial U.S. Approval: 1964
WARNING: SEVERE PERI-PROCEDURAL COMPLICATIONS, MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
- Severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events may occur with intra-hepatic administration of HEPZATO. Assess patients for these adverse reactions during and for 72 hours following administration of HEPZATO. (5.1)
- HEPZATO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the HEPZATO KIT REMS. (5.2)
- Myelosuppression with resulting severe infection, bleeding, or symptomatic anemia may occur with HEPZATO. Monitor hematologic laboratory parameters and delay additional cycles of HEPZATO therapy until blood counts have improved.(5.3)
Indications and Usage for Hepzato
HEPZATO is an alkylating drug indicated as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.(1)
Hepzato Dosage and Administration
HEPZATO, a component of the HEPZATO KIT, is administered by intra-arterial infusion into the hepatic artery (see instructions for use [IFU]). The recommended dose is 3 mg/kg based on ideal body weight (see Table 1), with a maximum absolute dose of 220 mg during a single HEPZATO treatment. (2.2). The drug is infused over 30 minutes followed by a 30-minute washout period (see IFU). Treatments should be administered every six (6) to eight (8) weeks but can be delayed until recovery from toxicities and as per clinical judgement. (2.3)
Dosage Forms and Strengths
For injection: HEPZATO includes 50 mg freeze-dried (lyophilized) melphalan powder per vial in five (5) single dose vials, intended for reconstitution with the supplied diluents. (3)
Contraindications
- Active intracranial metastases or brain lesions with a propensity to bleed
- Liver failure, portal hypertension, or known varices at risk for bleeding
- Surgery or medical treatment of the liver in the previous 4 weeks
- Active cardiac conditions including, but not limited to, unstable coronary syndromes (unstable or severe angina or myocardial infarction), worsening or new-onset congestive heart failure, significant arrhythmias, or severe valvular disease
- History of allergies or known hypersensitivity to melphalan or a component or material utilized within the HEPZATO KIT including natural rubber latex, heparin, and severe hypersensitivity to iodinated contrast not controlled by antihistamines and steroids (4)
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, have occurred in patients who received an intravenous (IV) formulation of melphalan. Immediately terminate hepatic arterial melphalan infusion for hypersensitivity reactions and administer supportive care. (5.4)
- Gastrointestinal disturbances such as nausea and vomiting, abdominal pain and diarrhea are common. (5.5)
- Carcinogenic/Mutagenic effects: Secondary malignancies, including acute nonlymphocytic leukemia, myeloproliferative syndrome, and carcinoma, have been reported in patients with cancer treated with alkylating drugs (including melphalan). Melphalan has been shown to cause chromatid or chromosome damage in humans. (5.6)
- Embryo-fetal toxicity: Can cause fetal harm. Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.7, 8.1, 8.3)
- Infertility: Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women and testicular suppression in men. (5.8)
Adverse Reactions/Side Effects
Most common (≥20%) adverse reactions or laboratory abnormalities are thrombocytopenia, fatigue, anemia, nausea, musculoskeletal pain, leukopenia, abdominal pain, neutropenia, vomiting, increased alanine aminotransferase, prolonged activated partial thromboplastin time, increased aspartate aminotransferase, increased alkaline phosphatase, and dyspnea. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Delcath at 1-833-632-0458 and www.Delcath.com or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch. (6)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2023
Full Prescribing Information
WARNING: PERI-PROCEDURAL COMPLICATIONS, MYELOSUPPRESSION
- Severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events may occur via hepatic intra-arterial administration of HEPZATO. Assess patients for these adverse reactions during and for at least 72 hours following administration of HEPZATO [see Warnings and Precautions (5.1)].
- HEPZATO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the HEPZATO KIT REMS [see Warnings and Precautions (5.2)].
- Myelosuppression with resulting severe infection, bleeding, or symptomatic anemia may occur with HEPZATO. Monitor hematologic laboratory parameters and delay additional cycles of HEPZATO therapy until blood counts have improved. [see Warnings and Precautions (5.1)]
1. Indications and Usage for Hepzato
HEPZATO for injection, as a component of the HEPZATO KIT, is indicated as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.
2. Hepzato Dosage and Administration
2.1 Important Pre-Treatment and Administration Information
HEPZATO is a component of the HEPZATO KIT Hepatic Delivery System [HDS]. Refer to the HEPZATO KIT Hepatic Delivery System Instructions for Use (IFU) for additional instructions including pre-infusion evaluation, hydration, premedication, anticoagulation, and supportive care.
Caution: The double balloon catheter component of the HDS contains natural rubber latex which may cause allergic reactions [see Contraindications (4)].
- Healthcare providers must complete the required HEPZATO KIT REMS training prior to administration of the HEPZATO KIT [see Warnings and Precautions (5.2)].
- Discontinue oral anticoagulation and drugs affecting platelet function prior to the procedure [see Warnings and Precautions (5.1)].
- Discontinue ACE-inhibitors, calcium channel blockers, or alpha-1-adrenergic blockers prior to the procedure [see Warnings and Precautions (5.1)].
- Conduct baseline hematologic testing. Administer intra-hepatic HEPZATO with the HEPZATO KIT only to patients with the following [see Warnings and Precautions (5.3)].
- Hemoglobin ≥ 10 g/dL
- Platelets ≥ 100,000/microliter
- Neutrophils > 2000/microliter
2.2 Recommended Dosage
- Administer HEPZATO via the HEPZATO KIT Hepatic Delivery System only to patients weighing 35 kg or greater due to potential size limitations with respect to percutaneous catheterization.
- HEPZATO, a component of the HEPZATO KIT, is administered by infusion into the hepatic artery (see IFU) every 6 to 8 weeks for up to 6 total infusions.
- The recommended HEPZATO dose is 3 mg/kg based on ideal body weight (IBW), as calculated per Table 1 below, with a maximum of 220 mg during a single treatment.
| Height | Ideal Body Weight | |
|---|---|---|
| Men | ≥ 152 cm | 52 kg + (0.75 kg/cm of height greater than 152 cm) |
| < 152 cm | 52 kg – (0.75 kg/cm of height less than 152 cm) | |
| Women | ≥ 152 cm | 49 kg + (0.67 kg/cm of height greater than 152 cm) |
| < 152 cm | 49 kg – (0.67 kg/cm of height less than 152 cm) |
2.3 Dosage Modifications for Adverse Reactions
A dosage reduction to 2 mg/kg is recommended for subsequent treatments for the following reasons:
- Grade 4 neutropenia of > 5 days duration despite growth factor support or associated with neutropenic fever;
- Grade 4 thrombocytopenia of > 5 days duration or associated with a hemorrhage that required a transfusion;
HEPZATO administered with the HEPZATO KIT should be discontinued if patients have life threatening or HEPZATO-related persistent toxicity that has not resolved to Grade 2 or less by 8 weeks following treatment.
2.4 Preparation and Administration
Refer to the HEPZATO KIT Hepatic Delivery System IFU for further details and instructions.
Reconstitute and dilute melphalan immediately prior to beginning intra-arterial infusion.
Reconstituted and diluted solutions of HEPZATO are unstable. No more than 60 minutes should elapse from reconstitution and completion of the intra-hepatic infusion of the diluted HEPZATO solution. A citrate derivative of melphalan has been detected in reconstituted HEPZATO in 30 minutes, and nearly 1% of labeled strength of melphalan hydrolyzes every 10 minutes when reconstituted HEPZATO is further diluted in 0.9% Sodium Chloride. A precipitate forms if the reconstituted solution is stored at 5°C. Do not refrigerate HEPZATO once reconstituted.
HEPZATO is a hazardous drug1. Follow applicable special handling and disposal procedures.
Reconstitution and Dilution Instructions:
- Rapidly (in 5 seconds or less) inject 10 mL of the supplied sterile diluent [see Dosage Forms and Strengths (3.0)] into the HEPZATO 50 mg vial using a sterile needle (20-gauge or larger) and syringe. The resulting solution will contain melphalan 5 mg/mL
- Immediately shake the vial vigorously until a clear solution is obtained. No more than five (5) seconds should elapse between the discharge of the syringe and the commencement of shaking.
- Immediately further dilute the required dose with the provided 0.9% sodium chloride injection, United States Pharmacopeia (USP), to a concentration not greater than 0.45 mg/mL, as follows
- HEPZATO doses up to 110 mg:
Dilute in 250 mL of 0.9% sodium chloride injection - HEPZATO doses 111 mg to 220 mg:
Divide the total dose equally into 2 and dilute each in 250 mL of 0.9% sodium chloride injection (for example, if the total dose is 200 mg, dilute 100 mg in each 250 mL 0.9% sodium chloride injection)
- HEPZATO doses up to 110 mg:
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulates and discolorations are noted, the product should not be used.
- Administer diluted HEPZATO intra-arterially as described in the IFU. Complete the infusion within 30 minutes, followed by a 30-minute washout period. Refer to the IFU for additional administration procedures.
3. Dosage Forms and Strengths
HEPZATO (melphalan) is supplied in the HEPZATO KIT that contains the following:
- Melphalan for injection: 5 single dose, clear glass vials for injection, containing 50 mg white to pale yellow lyophilized powder, intended for reconstitution with the supplied diluents
4. Contraindications
HEPZATO and the HEPZATO KIT are contraindicated in patients with:
- Active intracranial metastases or brain lesions with a propensity to bleed
- Liver failure, portal hypertension, or known varices at risk for bleeding
- Surgery or medical treatment of the liver in the previous 4 weeks
- Uncorrectable coagulopathy
- Inability to safely undergo general anesthesia, including active cardiac conditions including, but not limited to, unstable coronary syndromes (unstable or severe angina or myocardial infarction), worsening or new-onset congestive heart failure, significant arrhythmias, or severe valvular disease
- History of allergies or known hypersensitivity to melphalan
- History of allergies or known hypersensitivity to a component or material utilized within the HEPZATO KIT including
- History of allergy to natural rubber latex
- History of allergy or hypersensitivity to heparin or presence of heparin-induced thrombocytopenia (HIT)
- History of severe allergic reaction to iodinated contrast not controlled by premedication with antihistamines and steroids
5. Warnings and Precautions
5.1 Peri-Procedural Complications
Hemorrhage, hepatocellular injury, and thromboembolic events have been observed when HEPZATO has been administered via hepatic intra-arterial administration. Administration of HEPZATO requires general anesthesia and extracorporeal bypass of circulation which may cause life threatening or fatal adverse effects. Ensure the patient is euvolemic but do not overhydrate the patient. Monitor for these peri-procedural complications during the procedure and for at least 72 hours following the procedure.
To mitigate the risk of thromboembolic events, administer anticoagulation as described in the IFU during the procedure.
Due to the risk of bleeding, do not use in patients with uncorrectable coagulopathies and delay treatment with the HEPZATO KIT for at least 4 weeks after surgery or other medical procedure involving the liver. Platelets and clotting factors may be removed during the HEPZATO KIT procedure. Monitor platelets and coagulation parameters as described in the IFU. If life-threatening bleeding occurs during the procedure, reverse anticoagulation as described in the IFU and correct coagulopathy as appropriate. Discontinue anticoagulation with warfarin or other oral anticoagulants prior to the procedure; resume when hemostasis has been restored after the procedure, provided no bleeding complications have been observed. Refer to the Prescribing Information of the anticoagulant agent for bridging recommendations for anti-coagulation prior to surgical procedures. Discontinue drugs affecting platelet function such as aspirin, non-steroidal anti-inflammatory drugs, or other anti-platelet drugs one week before the procedure.
Patients with abnormal hepatic vascular (especially arterial supply) or biliary (especially re-implantation of bile duct) anatomy or gastric acid hypersecretion syndromes may be at increased risk of peri-procedural complications or other severe adverse reactions. Screen patients for a history of prior surgeries involving the bile duct to assess whether the patient is an appropriate candidate for HEPZATO KIT and monitor patients for adverse reactions following HEPZATO KIT administration.
Procedure-related reductions in blood pressure including severe hypotension can occur during the HEPZATO KIT procedure. Closely monitor blood pressure during the procedure. Patients may require fluid support and vasopressors. To reduce the risk of severe hypotension, assess hypothalamic-pituitary-adrenal axis function, and temporarily discontinue ACE-inhibitors, calcium channel blockers, or alpha-1-adrenergic blockers for at least 5 half-lives prior to treatment with the HEPZATO-KIT. If necessary, use other short-acting antihypertensive drugs to manage blood pressure during the peri-procedure period.
5.2 HEPZATO KIT REMS PROGRAM
The HEPZATO KIT is only available through a restricted program under a REMS, because of the risk of severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events defined in the REMS. The HEPZATO KIT should only be used by trained healthcare providers [see Warnings and Precautions (5.1)].
Important requirements of the HEPZATO KIT REMS include:
- Healthcare settings that dispense and administer HEPZATO KIT must be enrolled, certified, and comply with the REMS requirements.
- Certified healthcare facilities must ensure that healthcare providers who perform the Percutaneous Hepatic Perfusion (PHP) procedure are trained on the use of HEPZATO KIT and must only dispense HEPZATO when authorized to do so by the REMS.
- Certified healthcare facilities must ensure that patients are assessed for severe peri-procedural complications during the procedure and for at least 72 hours following the procedure.
Further information is available at www.HEPZATOKITREMS.com or contact Delcath Systems at 1-833-632-0457.
5.3 Myelosuppression
Hematologic adverse reactions, including thrombocytopenia, anemia, and neutropenia have been reported in patients treated with HEPZATO. The risk of hematologic adverse reactions may be increased in patients who have received prior chemotherapy, bone irradiation, or who have compromised bone marrow function.
In the 95 patients who received HEPZATO in the FOCUS trial, 68% had Grade 3 or 4 myelosuppression. A total of 55%, 33%, and 30% experienced Grade 3 or 4 thrombocytopenia, anemia, and neutropenia, respectively. Median time to thrombocyte nadir was 13 days (range: 3-33) after treatment with median recovery in 20 days (range: 4-29) after treatment. Median time to hemoglobin nadir was 10 days (range: 3-21) after treatment with median recovery in 13 days (range: 4-28) after treatment. Median time to neutrophil nadir was 11 days (range: 3-36) after treatment with median recovery in 17 days (range: 9-36) after treatment.
Monitor patients for severe infections, bleeding, and symptomatic anemia. Only administer HEPZATO in patients with platelets >100,000/microliter, hemoglobin ≥10.0 gm/dL and neutrophils >2,000/microliter. Administer transfusions or growth factors as appropriate [see Dosage and Administration (2.1)].
5.4 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received an intravenous (IV) formulation of melphalan. These reactions with melphalan are characterized by urticaria, pruritus, edema, skin rashes, and in some patients, tachycardia, bronchospasm, dyspnea, and hypotension. Hypersensitivity can occur in patients with or without prior exposure to IV or oral melphalan.
When a hypersensitivity reaction is observed, immediately terminate the hepatic arterial HEPZATO infusion and administer necessary supportive care [see Contraindications (4)].
Patients with a history of allergic reactions to iodinated contrast may experience hypersensitivity reactions, including anaphylaxis, during treatment with the HEPZATO KIT. Premedicate patients with a history of allergic reaction to iodinated contrast prior to treatment with HEPZATO KIT. Do not administer HEPZATO KIT in patients with a history of severe allergic reactions or anaphylaxis to iodinated contrast [see IFU, see Contraindications (4)].
5.5 Gastrointestinal Adverse Reactions
Gastrointestinal adverse reactions including nausea and vomiting, abdominal pain, and diarrhea are common, and occurred in 84% of patients treated with HEPZATO in the FOCUS trial. Administer a proton pump inhibitor the day prior to and the morning of the procedure. If anti-emetic treatment is required, pre-medicate with anti-emetic therapy in subsequent cycles.
5.6 Secondary Malignancies
Melphalan has been shown to cause chromatid or chromosome damage in humans. Secondary malignancies, including acute nonlymphocytic leukemia, myeloproliferative syndrome, and carcinoma, have been reported in patients with cancer treated with intravenous alkylating drugs including melphalan. Some patients also received other chemotherapeutic agents or radiation therapy. Precise quantification of the risk of acute leukemia, myeloproliferative syndrome, or carcinoma is not possible. Published reports of leukemia in patients who have received oral or IV melphalan (and other alkylating drugs) suggest that the risk of leukemogenesis increases with chronicity of treatment and with cumulative dose [see Nonclinical Toxicology (13.1)].
5.7 Embryo-Fetal Toxicity
Based on animal studies and its mechanism of action, melphalan can cause fetal harm when administered to a pregnant woman. Melphalan is genotoxic, targets actively dividing cells, and was embryolethal and teratogenic in rats. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with HEPZATO and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HEPZATO and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
5.8 Infertility
Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women, resulting in persistent amenorrhea in approximately 9% of patients. Reversible or irreversible testicular suppression has also been reported [see Use in Specific Populations (8.3)].
6. Adverse Reactions/Side Effects
Below are adverse reactions associated with HEPZATO KIT. Additional adverse reactions related to the procedure and/or medical device are described in further detail in the HEPZATO KIT IFU. The following clinically significant adverse reactions are described elsewhere in the labeling:
- Peri-procedural complications [see Warnings and Precautions (5.1)]
- Myelosuppression [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.5)]
- Secondary Malignancies [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug-device combination cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse drug reactions (ADRs) described in this section were identified from the FOCUS trial. FOCUS was a multicenter trial that evaluated HEPZATO (melphalan) administered via the HEPZATO KIT in patients with unresectable hepatic metastases from uveal melanoma. In the FOCUS trial, a total of 95 patients were enrolled into the HEPZATO KIT arm, of which 91 patients received treatment with HEPZATO.
Serious adverse reactions occurred in 45% of patients who received HEPZATO. Serious adverse reactions occurring in ≥ 2% of patients were thrombocytopenia (10%), neutropenia (8%), febrile neutropenia (7%), platelet count decreased (6%), leukopenia (4.2%), cardiac arrest (3.2%), neutrophil count decreased (2.1%), hypoxia (2.1%), pleural effusion (2.1%), pulmonary edema (2.1%), and deep vein thrombosis (2.1%). Fatal adverse reactions occurred in 3 (3.2%) patients who were treated with HEPZATO; these included cardiac arrest, acute hepatic failure and bacterial peritonitis.
HEPZATO was permanently discontinued due to adverse reactions in 18% of patients with neutropenia being the most common adverse reaction (3.2%) requiring permanent discontinuation.
Dose reductions due to an adverse reaction occurred in 14% of patients who received HEPZATO. Adverse reactions which required dose reductions occurring in ≥ 2% of patients were platelet count decreased (6%), neutropenia (4.2%), anemia (2.1%), and thrombocytopenia (2.1%).
Adverse reactions that required dosage interruption in ≥ 2% of patients who received HEPZATO were platelet count decreased (6%), neutropenia (5%), thrombocytopenia (3.2%), anemia (3.2%) and febrile neutropenia (2.1%).
The most common (≥20%) adverse reactions or laboratory abnormalities reported in patients treated with HEPZATO were thrombocytopenia (65%), fatigue (65%), anemia (63%), nausea (57%), musculoskeletal pain (46%), leukopenia (46%), abdominal pain (39%), neutropenia (35%), vomiting (35%), increased alanine aminotransferase (32%), prolonged activated partial thromboplastin time (28%), increased aspartate aminotransferase (28%), increased blood alkaline phosphatase (27%), and dyspnea (23%).
Table 2 and Table 3 summarize adverse reactions and laboratory abnormalities, respectively, that occurred in FOCUS.
|
1Represents a composite of multiple, related preferred terms |
||
| All Adverse Reactions N=95 | ||
| All Grades (%) | Grades 3 or 4 (%) | |
| Gastrointestinal disorders | ||
| Nausea | 57 | 0 |
| Abdominal Pain1 | 39 | 1 |
| Vomiting1 | 35 | 0 |
| Diarrhea1 | 17 | 1 |
| General disorders | ||
| Fatigue1 | 65 | 0 |
| Pyrexia1 | 16 | 0 |
| Musculoskeletal And Connective Tissue Disorders | ||
| Musculoskeletal Pain1 | 46 | 1 |
| Groin Pain | 11 | 0 |
| Respiratory disorders | ||
| Dyspnea1 | 23 | 2 |
| Cough1 | 15 | 0 |
| Nervous system disorders | ||
| Headache1 | 19 | 0 |
| Lethargy | 12 | 0 |
| Dizziness1 | 11 | 0 |
| Injury and procedural complications | ||
| Contusion | 17 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 16 | 0 |
| Vascular disorders | ||
| Hemorrhage1 | 15 | 1 |
| Hypotension1 | 13 | 3 |
|
a Represents a composite of multiple, related preferred terms |
||
| Laboratory Abnormality | All Laboratory Abnormalities N=95 | |
| All Grades (%) | Grades 3 or 4 (%) |
|
| Platelets decreaseda | 65 | 55 |
| Hemoglobin decreaseda | 63 | 33 |
| Leukocytes decreaseda | 46 | 34 |
| Neutrophils decreaseda | 35 | 30 |
| Alanine aminotransferase increased | 32 | 3 |
| International normalized ratio increased | 31 | 8 |
| Activated partial thromboplastin time prolonged | 28 | 8 |
| Aspartate aminotransferase increased | 28 | 4 |
| Blood alkaline phosphatase increased | 27 | 2 |
| Calcium decreased | 13 | 3 |
| Troponin I increased | 13 | 2 |
| Blood bilirubin increased | 11 | 3 |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal studies and its mechanism of action, melphalan can cause fetal harm when administered to a pregnant woman, including teratogenicity and/or embryo-fetal lethality [see Clinical Pharmacology (12.1)]. Melphalan is a genotoxic drug and can cause chromatid or chromosome damage in humans [see Nonclinical Toxicology (13.1)]. In animal studies, melphalan was embryolethal and teratogenic in rats at doses below the recommended clinical doses [see Data]. Advise a pregnant woman of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the United States general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
Adequate animal studies have not been conducted with IV melphalan. Melphalan was embryolethal and teratogenic in rats following oral administration of 6 to 18 mg/m2/day for ten (10) days (0.05 to 0.16 times the recommended clinical dose of 3 mg/kg or 111 mg/m2/day) and intraperitoneal administration of 18 mg/m2 (0.16 times the highest recommended clinical dose). Malformations resulting from melphalan administration included alterations of the brain (underdevelopment, deformation, meningocele, and encephalocele) and eye (anophthalmia and microphthalmos), reduction of the mandible and tail, and hepatocele (exomphaly).
8.2 Lactation
Risk Summary
It is not known whether melphalan is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing children from melphalan, breastfeeding is not recommended during treatment with melphalan and for one week after the last dose.
8.3 Females and Males of Reproductive Potential
Melphalan can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to initiating HEPZATO [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with HEPZATO and for 6 months after the last dose.
Males
HEPZATO administration may damage spermatozoa and testicular tissue, resulting in possible genetic fetal abnormalities. Advise males with female partners of reproductive potential to use effective contraception during treatment with HEPZATO and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].
10 OVERDOSE
No information on melphalan overdosage is available following administration of HEPZATO. Overdoses resulting in death have been reported following treatment with high intravenous (IV) doses of melphalan.
Overdoses via the IV route, including doses up to 290 mg/m2 (approximately 7.5 mg/kg IBW), have produced the following symptoms: severe nausea and vomiting, decreased consciousness, convulsions, muscular paralysis, and cholinomimetic effects. Severe mucositis, stomatitis, colitis, diarrhea, and hemorrhage of the gastrointestinal tract occur at high IV doses (>100 mg/m2 or approximately 2.6 mg/kg IBW). Elevations in liver enzymes and veno-occlusive disease occur infrequently. Significant hyponatremia caused by an associated inappropriate secretion of antidiuretic hormone syndrome has been observed. Nephrotoxicity and adult respiratory distress syndrome have been reported.
The principal toxic effect is bone marrow suppression. Hematologic parameters should be closely followed for three (3) to six (6) weeks. General supportive measures together with appropriate blood transfusions and antibiotics should be instituted as deemed necessary by the physician. General supportive measures, together with appropriate blood and platelet transfusions, should be instituted if necessary and consideration given to hospitalization, antibiotic cover, and the use of hematological growth factors.
This drug is not removed from systemic plasma to any significant degree by hemodialysis or hemoperfusion.
11. Hepzato Description
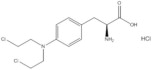
Melphalan, is a bifunctional alkylating drug that is active against selected human neoplastic diseases. Melphalan is available as melphalan hydrochloride salt. The chemical name of melphalan hydrochloride is 4-[bis(2-chloroethyl)amino]-L-phenylalanine hydrochloride. The molecular formula is C13H18Cl2N2O2.HCl and the molecular weight is 341.67.
Melphalan is practically insoluble in water and has a pKa1 of ~2.5.
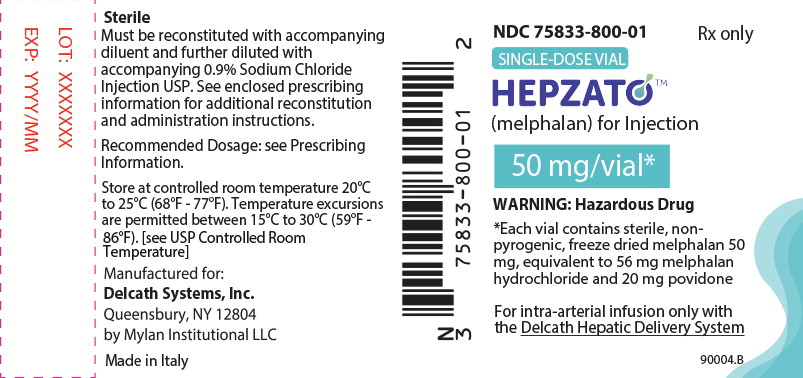
HEPZATO, for injection, is supplied as a sterile, nonpyrogenic, freeze-dried white to pale yellow freeze-dried cake/ powder. Each single dose vial contains melphalan 50 mg, equivalent to 56 mg of melphalan hydrochloride and 20 mg povidone.
HEPZATO (melphalan) is reconstituted using the sterile diluent provided. Each vial of sterile diluent contains sodium citrate 0.2 g, propylene glycol 6.0 mL, ethanol (96%) 0.52 mL, and water for injection to a total of 10 mL.
HEPZATO (melphalan) for use with the hepatic delivery system is administered intra-arterially.
12. Hepzato - Clinical Pharmacology
12.1 Mechanism of Action
Melphalan is an alkylating drug of the bischloroethylamine type. As a result, its cytotoxicity appears to be related to the extent of its interstrand cross-linking with DNA, probably by binding at the N7 position of guanine. It is active against both resting and rapidly dividing tumor cells.
12.3 Pharmacokinetics
Geometric mean of systemic melphalan maximum concentration (Cmax) is 2.4 (%CV 3.0) mcg/mL and the AUC0-last was 1.8 (%CV 1.1) mcg*hr/mL.
The melphalan median (range) time to Cmax (Tmax) is 0.57 (0.05 – 1.18) hours following administration of HEPZATO.
Distribution
The melphalan plasma protein binding is approximately 78% following administration of HEPZATO. Serum albumin accounts for approximately 40% to 60% and α1-acid glycoprotein approximately 20% of the plasma protein binding.
Elimination
The median terminal elimination phase half-life of 1.07 hours that is consistent with IV melphalan administration.
Excretion:
Liver uptake and removal of melphalan by isolation of hepatic venous blood and subsequent filtration by HDS are the two main processes for reducing the amount of melphalan that is available systemically following administration of HEPZATO. HDS reduced systemic melphalan exposure with a mean (SD) filter efficiency of 82.7% (14.4%) for the total filtration period.
Systemic melphalan is eliminated by renal excretion of parent drug and metabolites.
Specific Populations
No clinically significant differences in the pharmacokinetics of melphalan were observed based on body weight (43 - 150 kg), creatinine clearance (> 50 mL/min), or hepatic parameters (ALT (7 - 157 IU/L), AST (11 - 90 IU/L), or bilirubin (0.06 - 1.5 mg/dL) following administration of HEPZATO KIT.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate and well-controlled carcinogenicity studies have not been conducted in animals. However, intraperitoneal (IP) administration of melphalan in rats (5.4 to 10.8 mg/m2) and in mice (2.25 to 4.5 mg/m2) 3 times per week for 6 months followed by 12 months post-dose observation produced peritoneal sarcoma and lung tumors, respectively.
Intramuscular administration of melphalan at 6 and 60 mg/m2 produced structural aberrations of the chromatid and chromosomes in bone marrow cells of Wistar rats.
14. Clinical Studies
Study in Patients with Uveal Melanoma
The efficacy of HEPZATO in hepatic-dominant metastatic uveal melanoma was based on the results from 91 patients who received HEPZATO via the HEPZATO KIT in the FOCUS Study (NCT02678572), a multicenter, open-label trial. To be eligible for enrollment, patients were required to have metastatic uveal melanoma with metastases predominately involving the liver (liver dominant). Limited extrahepatic disease in the bone, subcutaneous sites, lymph nodes, or lung was permitted if the life-threatening component of the uveal melanoma was in the liver and the extrahepatic disease was amenable to resection or radiation and had a defined treatment plan. Patients with metastases in more ≥ 50% of the liver parenchyma, unable to undergo general anesthesia, ECOG ≥2, platelets < 100,000/microliter, absolute neutrophil count < 1,500/microliter, hemoglobin < 10 gm/dL, Child-Pugh Class B or C cirrhosis, or hepatitis B or C infection were excluded.
Patients received 3 mg/kg of melphalan based on ideal body weight (IBW, maximum total dose of 220 mg) administered intraarterially using the Hepatic Delivery System (HDS) every 6-8 weeks for up to 6 infusions. The median number of infusions administered per patient was 4 (range: 1-6). Thirty-seven percent (37%) of the 91 patients treated received the maximum of six infusions of treatment.
The major efficacy outcome measures were objective response rate (ORR) and duration of response (DoR) using computed tomography (CT) or magnetic resonance imaging (MRI) assessed by an independent central review committee (IRC) using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The median age of patients was 61 years (range 20 to 78), 52% were female, 95% were White, 5% unavailable, and all patients had Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Ninety-five percent (95%) of enrolled patients had either 2 or 3 hepatic lesions. Seventy-nine percent (79%) of patients had <25% liver involvement. Thirty percent of the treated patients had extra-hepatic lesions, of which 20% had 1 extrahepatic lesion and 10% had 2 or more; overall 12% of patients had lung, 11% soft tissue/subcutaneous, 5% lymph node, and 4% had bone involvement. Forty-three percent (43%) of patients underwent prior therapy for metastatic disease, including systemic therapy (25%), other surgeries or procedures (14%), and radiation (11%).
The efficacy results of HEPZATO treatment are summarized in Table 4.
|
1 Clopper-Pearson method 2 Kaplan Meier method 3 Brookmeyer and Crowley method 4 Based on observed duration of response |
|
| HEPZATO
(N=91) |
|
| Objective Response Rate | |
| ORR (95% CI)1 | 36.3% (26.4, 47.0) |
| Complete Response | 7.7% |
| Partial Response | 28.6% |
| Duration of response (months) | |
| Number of Responders | n= 33 |
| Median2 (months) (95% CI)3 | 14.0 (8.3, 17.7) |
| % Responder with DoR≥6 months4 | 70 % |
| % Responder with DoR≥12 months4 | 30 % |
16. How is Hepzato supplied
How Supplied
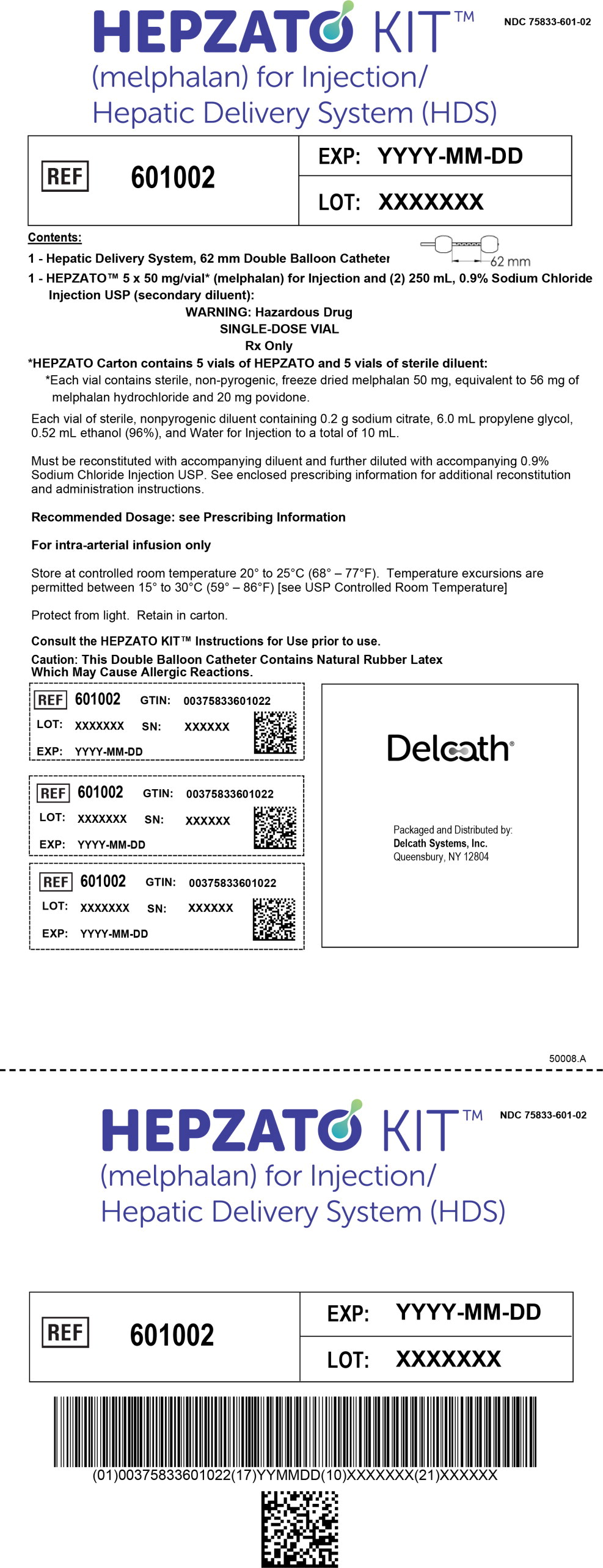
The HEPZATO KIT includes the HEPZATO 5 x 5 Drug Pack and the Hepatic Delivery System (HDS). Each 5 x 5 HEPZATO KIT Drug Pack includes HEPZATO (melphalan) and diluents for reconstitution and dilution:
- Each vial of HEPZATO contains 50 mg melphalan for injection supplied as a sterile, nonpyrogenic, freeze-dried cake/powder in a carton containing 5 single-dose, glass vials (NDC 75833-800-01).
- Each vial of sterile diluent contains sodium citrate 0.2 g, propylene glycol 6.0 mL, ethanol (96%) 0.52 mL, and water for injection to a total of 10 mL in a carton containing 5 single-dose, glass vials for reconstitution (NDC 75833-700-01).
- Each of two 250 mL plastic containers of 0.9% sodium chloride injection USP (NDC 0264-7800-20).
HEPZATO (melphalan) for injection must only be administered with the HDS device supplied with the HEPZATO KIT and components specified by Delcath Systems, Inc, in the IFU [see Dosage and Administration (3)].
Storage and Handling
HEPZATO for injection and its associated diluents including 0.9% sodium chloride must be stored at controlled room temperature 20°C to 25°C (68°F to 77°F). Temperature excursions are permitted between 15°C- 30°C (59°F-86°F) [see USP Controlled Room Temperature].
The Hepatic Delivery System components may be stored at room temperature.
Melphalan is a hazardous drug. Follow applicable special handling and disposal procedures.1
HEPZATO is light sensitive. Retain in original carton until use.
HEPZATO (melphalan) for injection
Manufactured for:
Delcath Systems, Inc.
Queensbury, NY 12804
by Mylan Institutional LLC
Made in Italy
HEPZATO KIT (melphalan for Injection/Hepatic Delivery System)
Packaged and Distributed by:
Delcath Systems, Inc.
Queensbury, NY 12804
17. Patient Counseling Information
Advise patients or their caregivers of the following risks of the HEPZATO KIT:
Peri-Procedural Complications
- Advise patients of the severe procedural risks associated with administration of melphalan using the HEPZATO KIT including hemorrhage, hepatic injury, and thromboembolic events.
- Advise patients that they will be monitored in a hospital setting following each treatment.
- Advise patients taking anti-coagulant and anti-hypertensive medications that these may need to be discontinued prior to treatment with HEPZATO KIT [see Warnings and Precautions (5.1)].
Myelosuppression
- Advise patients to immediately contact their healthcare provider for a fever, bruising, or bleeding. Advise patients of the need for monitoring of blood counts [see Warnings and Precautions (5.3)].
Hypersensitivity
- Inform patients of the signs and symptoms of hypersensitivity. Advise patients to immediately report symptoms of hypersensitivity [see Warnings and Precautions (5.4)].
Latex Allergy
- The double balloon catheter component of the HEPZATO KIT Hepatic Delivery System contains natural rubber latex which may cause allergic reactions in latex-sensitive individuals [see Contraindications (4)].
Gastrointestinal
- Advise patients to report symptoms of nausea, vomiting and diarrhea, so that appropriate antiemetic and/or antidiarrheal medications can be administered [see Warnings and Precautions (5.5)].
Secondary Malignancies
- Advise patients that treatment with HEPZATO has the potential long-term risk of secondary malignancy [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to a fetus [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with HEPZATO and for 6 months after the last dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking HEPZATO [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
- Advise males with female partners of reproductive potential to use effective contraception during treatment with HEPZATO and for 3 months after the last dose [see Use in Specific Populations (8.3)].
Infertility
- Inform both females and males of reproductive potential about the risk of infertility [see Warnings and Precautions (5.8) and Use in Specific Populations (8.3)].
HEPZATO (melphalan) for Injection
- Manufactured for:
- Delcath Systems, Inc.
- Queensbury, NY 12804
- by Mylan Institutional LLC
- Made in Italy
HEPZATO KIT (melphalan for Injection/Hepatic Delivery System)
- Packaged and Distributed by:
- Delcath Systems, Inc.
- Queensbury, NY 12804
© Copyright, 2023 Delcath Systems, Inc. All rights reserved.
120070.A
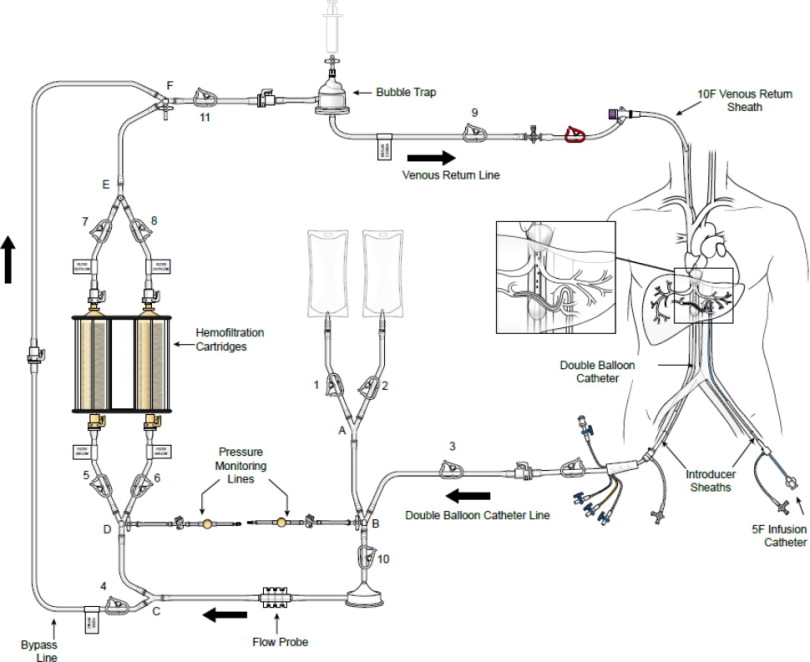
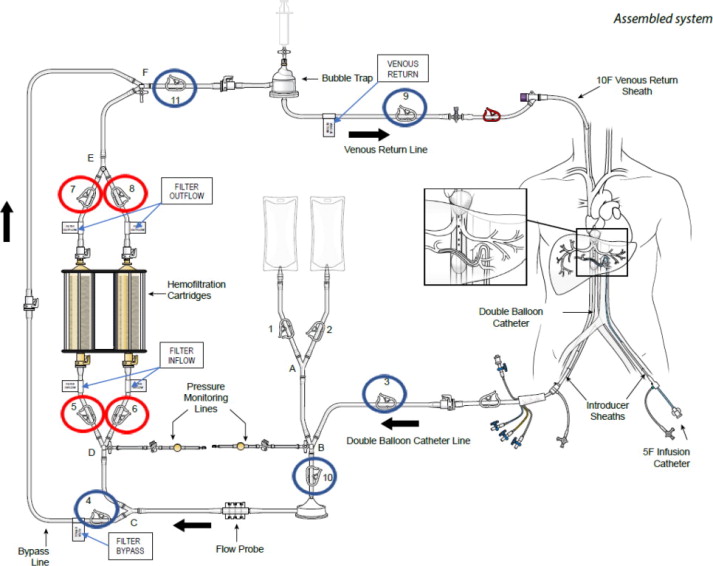
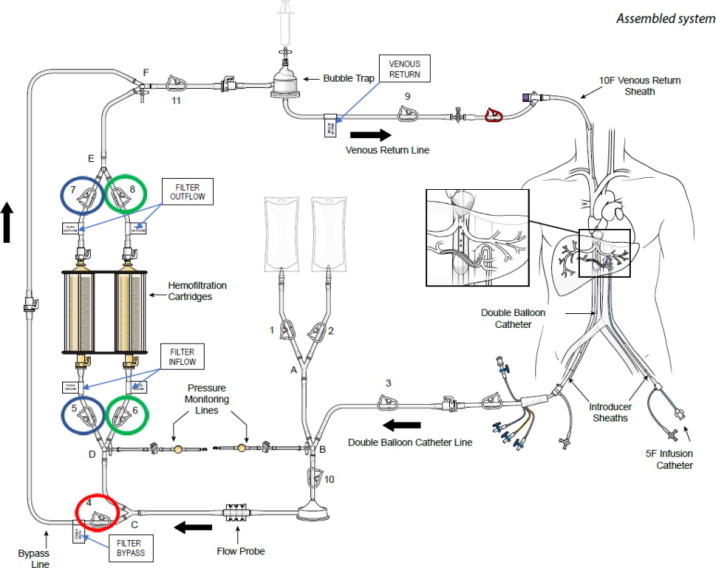
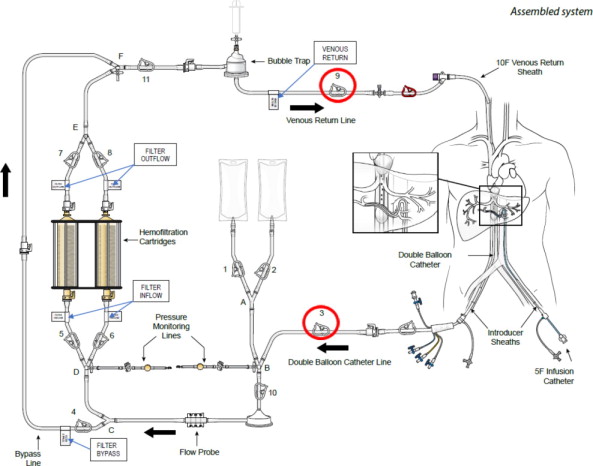
(a) ASSEMBLED SYSTEM – FIGURE 1
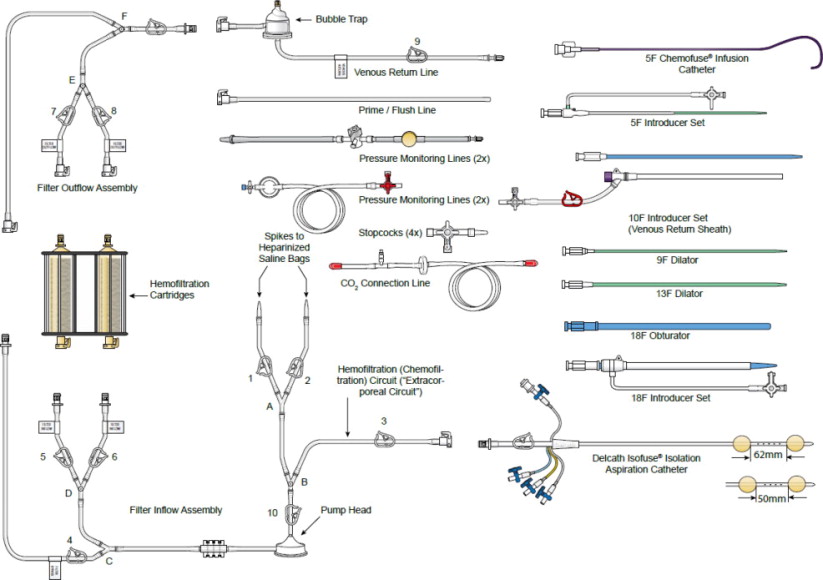
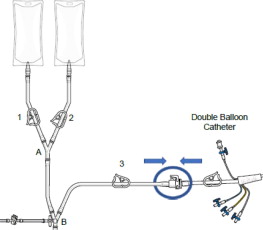
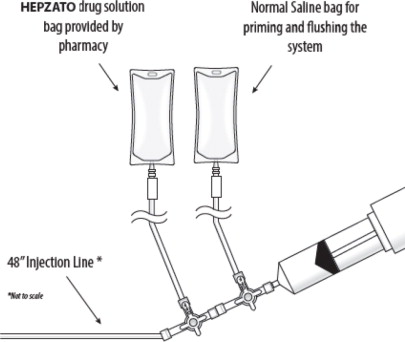
(b) SUPPLIED DISPOSABLE COMPONENTS – FIGURE 2
HEPZATO KIT™
HEPZATO (melphalan) for Injection/
Hepatic Delivery System
COMPLETE REQUIRED REMS TRAINING BEFORE USING THIS DEVICE FOR THE FIRST TIME. ENSURE YOU COMPLETELY READ AND UNDERSTAND THE INSTRUCTIONS FOR USE.
(c) DESCRIPTION OF SYSTEM COMPONENTS
HEPZATO KIT™ consists of a closed circuit of catheters and drug-specific filters utilized to deliver Hepzato (melphalan) to the hepatic artery and to lower the concentration of melphalan in the blood before it is returned to systemic circulation. A schematic overview of how the Hepatic Delivery System components work together is presented in Figure 1: Assembled System. The system is designed to be used with a Medtronic Bio-Console® 560 Speed Controller System and TX50P Flow Transducer.
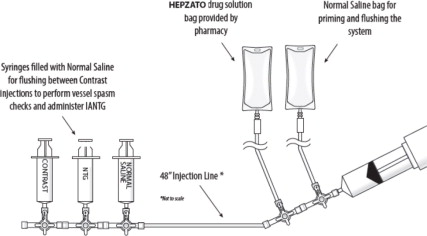
1. Double Balloon Catheter (DBC) -- 16F (shaft) polyurethane double balloon catheter that is placed in the retro-hepatic inferior vena cava to isolate the hepatic venous blood and transport it to the Extracorporeal Hemofiltration Circuit for filtration. The catheter has one large (central) drainage lumen and four accessory ports. Due to variation in the length of a patient's retro-hepatic segment of the inferior vena cava and relative positions of hepatic and renal veins, the Double Balloon Catheter is available in two different balloon configurations: 50 mm or 62 mm between the two balloons.
Using pre-operative computed tomography (CT) imaging, or by performing an inferior vena cavogram prior to placement of the Double Balloon Catheter, estimate the length of the retro-hepatic segment of the inferior vena cava and the relative positions of hepatic and renal veins in order to determine the optimum Double Balloon Catheter balloon spacing: 50mm or 62mm.
Two of the accessory ports are used to inflate low-pressure occlusion balloons, which are inflated independently to occlude the inferior vena cava above and below the hepatic veins. When inflated, the cephalic (superior – blue port) balloon obstructs the inferior vena cava above the hepatic veins and the caudal (inferior – yellow port) balloon obstructs the inferior vena cava below the hepatic veins, thus isolating hepatic venous blood in the fenestrated segment between the balloons.
The large drainage lumen with a quick connect fitting is a conduit to the fenestrations between the two occlusion-balloons. The fenestrations allow the hepatic venous blood to flow into the drainage lumen and exit the catheter at the proximal end.
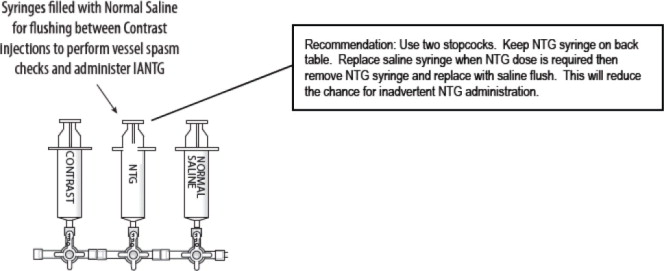
The third accessory (translucent) port labeled “CONTRAST” is for injections of iodinated contrast medium through the fenestrations, to check catheter position.
The fourth accessory port (white) is used for over-the-guidewire (OTW) introduction and positioning of the catheter in the retro-hepatic inferior vena cava. This lumen also has a small port opening along the catheter shaft positioned inferior to the caudal balloon and exits at the distal tip, to allow inferior vena cava blood, proximal to the caudal balloon, to bypass the occluded segment of the inferior vena cava and flow into the right atrium.
2. Accessory Pack
- 9F and 13F Dilator Set --These over-the-wire dilators are used to widen the subcutaneous space and venous entry site in preparation for the placement of the 18F Introducer Set.
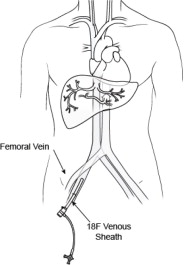
- 18F Introducer Set (Sheath and Dilator) -- The 18F introducer sheath and coaxial dilator are to be placed over a wire; the dilator is removed, and the sheath is available for the insertion of the Double Balloon Catheter or the 18F Obturator.
- 18F Obturator -- An 18F obturator is used to occlude and support the 18F sheath lumen when it is not in use, and upon removal of the Double Balloon Catheter at the end of the procedure.
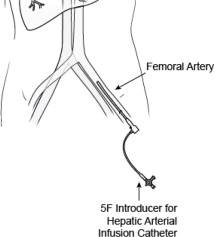
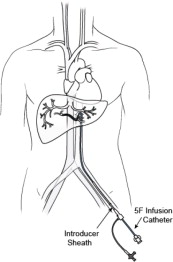
- 5F Introducer Set (Sheath and Dilator) -- A 5F hemostasis sheath is used to facilitate the introduction of the 5F Infusion Catheter through the femoral artery.
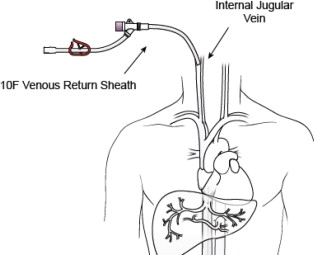
- 10F Introducer Set (Venous Return Sheath) -- A 10F sheath used to return the filtered hepatic venous blood through the internal jugular vein. A 3-way high-flow stopcock is included as part of the 10F Introducer Set. The high-flow stopcock is attached to the Venous Return Sheath and then to the male connector of the Extracorporeal Hemofiltration Circuit, if required. This sheath may also be used for hydration.
3. 5F Infusion Catheter -- 5F arterial catheter is used to deliver Hepzato into the proper hepatic artery or it can be used to coaxially introduce a microcatheter, if, at the discretion of the Interventional Radiologist, a microcatheter is preferred for selective catheter tip placement for the drug infusion. The following microcatheters have been qualified for use with the Hepzato KIT™ - select one of the microcatheters below. See microcatheter manufacturer's Instructions for Use. These microcatheters are NOT PROVIDED by Delcath:
- Merit Maestro (Merit Medical Systems, Inc., So. Jordan, UT, USA)
- Boston Scientific Renegade Hi-Flo (Boston Scientific Corp., Natick, MA, USA)
- Terumo Progreat (Terumo Medical Corp., Somerset, NJ, USA)
4. Delcath Hemofiltration Dual Filter Cartridge -- One single-use Dual Filter Cartridge designed with the filter cartridges arranged in parallel to lower the concentration of Hepzato (melphalan) in systemic circulation. The cartridge frame comes with a built-in pole clamp.
Filter mechanism of action: The filter is composed of activated carbon for adsorption and removal of HEPZATO. The pore structure of the filter media along with its particle size and shape contribute to rapid HEPZATO adsorption where large quantities (70 cc/100 g of carbon with melphalan) are adsorbed during a first pass through the filter Flow rates should be between 0.4 Liters/minute to 0.8 Liters/minute, to ensure the appropriate removal of the HEPZATO from the blood during the 30-minute infusion period and continue to remove residual drug during the 30-minute wash out period.
5. Extracorporeal Hemofiltration Circuit (EFC) -- The Extracorporeal Hemofiltration Circuit is used to transport the hepatic venous blood, which has been isolated by the Double Balloon Catheter and aspirated into the fenestration lumen, through the Hemofiltration Cartridges and back to the patient through the Venous Return Sheath. Connections are provided for infusion of normal saline. This circuit includes:
- Medtronic BP-50 Bio-Pump® Centrifugal Pump (“Pump Head”), a disposable pump head to be used with a pump console manufactured by Medtronic, Inc. - see manufacturer's Instructions for Use for Pump Head. (Note: The Medtronic, Inc. Bio-Console 560 (extracorporeal blood pumping) System is required for use with the Hepatic Delivery System: This is NOT PROVIDED by Delcath).
- Medtronic Bio-Probe® DP-38P blood flow monitoring insert (“Flow Probe”), a disposable flow probe to be used with a blood flow monitoring transducer manufactured by Medtronic, Inc. - see manufacturer's Instructions for Use for Flow Probe. The Flow Probe is used to measure the rate of blood flow during the procedure. (Note: The Medtronic Bio-Probe TX50P blood flow monitoring transducer is required for use with the Hepatic Delivery System: This is NOT PROVIDED by Delcath).
- Prime/Flush Line to be used to prime the system and flush the filter after system is fully de-bubbled (de-gassed).
- Pressure Monitoring Lines allow measuring the positive and negative pressure pre- and post- pump head.
- High Flow Stopcocks to allow the CO2 Connection Line and the pressure monitoring lines to be attached to the assembled circuit.
- Venous Return Line to be attached post system prime and flush so that the circuit can be attached to the 10F Venous Return Sheath.
6. Carbon Dioxide (CO2) Connection Line -- The CO2 Connection Line is used to deliver sterile CO2 gas to the Hemofiltration Cartridges to aid in priming/debubbling the filter cartridge, prior to the start of the procedure. The CO2 Line has no patient contact.
| WARNING
Only the components provided in the HEPZATO KIT or specified by Delcath Systems, Inc. in the “not included” box below are to be used to create the circuit. There should be no substitutions. The circuit has not been validated for use with other components. Do not disassemble the components provided in the Hepatic Delivery System as this may damage the components. |
| NOT INCLUDED: |
|
(d) INDICATIONS FOR USE
HEPZATO KIT™, containing HEPZATO for injection, is indicated as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.
| (e) WARNING: PERI-PROCEDURAL COMPLICATIONS, MYELOSUPPRESSION |
| Severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events may occur via hepatic intra-arterial administration of HEPZATO. Assess patients for these adverse reactions during and for at least 72 hours following administration of HEPZATO.
HEPZATO KIT is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the HEPZATO KIT REMS. Myelosuppression with resulting severe infection, bleeding, or symptomatic anemia may occur with HEPZATO. Monitor hematologic laboratory parameters and delay additional cycles of HEPZATO therapy until blood counts have improved. |
(f) CONTRAINDICATIONS
HEPZATO KIT is contraindicated in patients with:
- Active intracranial metastases or brain lesions with a propensity to bleed
- Liver failure, portal hypertension, or known varices at risk for bleeding
- Surgery or medical treatment of the liver in the previous 4 weeks
- Uncorrectable coagulopathy
- Inability to safety undergo general anesthesia, including active cardiac conditions including, but not limited to, unstable coronary syndromes (unstable or severe angina or myocardial infarction), worsening or new-onset congestive heart failure, significant arrhythmias, or severe valvular disease
- History of allergies or known hypersensitivity to melphalan
- History of allergies or known hypersensitivity to a component or material utilized within the HEPZATO KIT including:
- History of allergy to natural rubber latex
- History of allergy or hypersensitivity to heparin or presence of heparin-induced thrombocytopenia (HIT)
- History of severe allergic reaction to iodinated contrast not controlled by premedication with antihistamines and steroids
(g) WARNINGS AND PRECAUTIONS
PLEASE CAREFULLY READ AND UNDERSTAND THE LIST OF WARNINGS AND PRECAUTIONS BELOW AS SERIOUS INJURY, ILLNESS OR DEATH OF THE PATIENT CAN OCCUR IF THESE WARNINGS AND PRECAUTIONS ARE NOT PROPERLY FOLLOWED.
Peri-Procedural Complications
Hemorrhage, hepatocellular injury, and thromboembolic events have been observed when HEPZATO has been administered via hepatic intra-arterial administration. Administration of HEPZATO KIT requires general anesthesia and extracorporeal bypass of circulation which may cause life threatening or fatal adverse effects. Ensure the patient is euvolemic but do not overhydrate the patient. Monitor for these peri-procedural complications during the procedure and for at least 72 hours following the procedure.
To mitigate the risk of thromboembolic events, administer anticoagulation as described in the IFU during the procedure.
Due to the risk of bleeding, do not use in patients with uncorrectable coagulopathies and delay treatment with the HEPZATO KIT for at least 4 weeks after surgery or other medical procedure involving the liver. Platelets and clotting factors may be removed during the HEPZATO KIT procedure. Monitor platelets and coagulation parameters as described in the IFU. If life-threatening bleeding occurs during the procedure, reverse anticoagulation as described in the IFU and correct coagulopathy as appropriate. Discontinue anticoagulation with warfarin or other oral anticoagulants prior to the procedure until hemostasis has been restored after the procedure and no bleeding complications have been observed. Refer to the Prescribing Information of the anticoagulant agent for bridging recommendations for anti-coagulation prior to surgical procedures. Discontinue drugs affecting platelet function such as aspirin, non-steroidal anti-inflammatory drugs, or other anti-platelet drugs one week before the procedure.
Patients with abnormal hepatic vascular (especially arterial supply) or biliary (especially re-implantation of bile duct) anatomy or gastric acid hypersecretion syndromes may be at increased risk of peri-procedural complications or other severe adverse reactions. Screen patients for a history of prior surgeries involving the bile duct to assess whether the patient is an appropriate candidate for HEPZATO KIT and monitor patients for adverse reactions following HEPZATO KIT administration.
Procedure-related reductions in blood pressure including severe hypotension can occur during the HEPZATO KIT procedure. Closely monitor blood pressure during the procedure. Patients may require fluid support and vasopressors. To reduce the risk of severe hypotension, assess hypothalamic-pituitary-adrenal axis function, and temporarily discontinue ACE-inhibitors, calcium channel blockers, or alpha-1-adrenergic blockers for at least 5 half-lives prior to treatment with the HEPZATO-KIT. If necessary, use other short-acting antihypertensive drugs to manage blood pressure during the peri-procedure period.
HEPZATO KIT REMS Program
The HEPZATO KIT is only available through a restricted program under a REMS, because of the risk of severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events defined in the REMS. The HEPZATO KIT should only be used by trained healthcare providers [see HEPZATO USPI Warnings and Precautions (5.2)].
Important requirements of the HEPZATO KIT REMS include:
Healthcare settings that dispense and administer HEPZATO KIT must be enrolled, certified, and comply with the REMS requirements.
Certified healthcare facilities must ensure that healthcare providers who perform the Percutaneous Hepatic Perfusion (PHP) procedure are trained on the use of HEPZATO KIT and must only dispense HEPZATO when authorized to do so.
Certified healthcare facilities must ensure that patients are assessed for severe peri-procedural complications during the procedure and for at least 72 hours following the procedure.
Further information is available at www.HEPZATOKITREMS.com or contact Delcath Systems at 1-833-632-0457.
Myelosuppression
Hematologic adverse reactions, including thrombocytopenia, anemia, and neutropenia have been reported in patients treated with HEPZATO. The risk of hematologic adverse reactions may be increased in patients who have received prior chemotherapy, bone irradiation, or who have compromised bone marrow function.
In the 95 patients who received HEPZATO KIT in the FOCUS trial, 68% had Grade 3 or 4 myelosuppression. A total of 55%, 33%, and 30% experienced Grade 3 or 4 thrombocytopenia, anemia, and neutropenia, respectively. Median time to thrombocyte nadir was 13 days (range: 3-33) after treatment with median recovery in 20 days (range: 4-29) after treatment. Median time to hemoglobin nadir was 10 days (range: 3-21) after treatment with median recovery in 13 days (range: 4-28) after treatment. Median time to neutrophil nadir was 11 days (range: 3-36) after treatment with median recovery in 17 days (range: 9-36) after treatment.
Monitor patients for severe infections, bleeding, and symptomatic anemia. Only administer HEPZATO in patients with platelets >100,000/microliter, hemoglobin ≥10.0 gm/dL and neutrophils >2,000/microliter. Administer transfusions or growth factors as appropriate [see HEPZATO USPI Dosage and Administration (2.1)].
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received an intravenous (IV) formulation of melphalan. These reactions with melphalan are characterized by urticaria, pruritus, edema, skin rashes, and in some patients, tachycardia, bronchospasm, dyspnea, and hypotension. Hypersensitivity can occur in patients with or without prior exposure to IV or oral melphalan.
When a hypersensitivity reaction is observed, immediately terminate the hepatic arterial melphalan infusion and administer necessary supportive care [see HEPZATO USPI Contraindications (4), and Adverse Reactions (6.1)].
Patients with a history of allergic reactions to iodinated contrast may experience hypersensitivity reactions, including anaphylaxis, during treatment with the HEPZATO KIT. Premedicate patients with a history of allergic reaction to iodinated contrast prior to treatment with HEPZATO KIT. Do not administer HEPZATO KIT in patients with a history of severe allergic reactions or anaphylaxis to iodinated contrast [see IFU contraindications and HEPZATO USPI Contraindications (4)].
Gastrointestinal Adverse Reactions
Gastrointestinal adverse reactions including nausea and vomiting, abdominal pain, and diarrhea are common, and occurred in 84% of patients treated with HEPZATO KIT in the FOCUS trial. Administer a proton-pump inhibitor (PPI) the day prior to and the morning of the procedure. If anti-emetic treatment is required, pre-medicate with anti-emetic therapy in subsequent cycles.
Secondary Malignancies
Melphalan has been shown to cause chromatid or chromosome damage in humans. Secondary malignancies, including acute nonlymphocytic leukemia, myeloproliferative syndrome, and carcinoma, have been reported in patients with cancer treated with intravenous alkylating drugs including melphalan. Some patients also received other chemotherapeutic agents or radiation therapy. Precise quantification of the risk of acute leukemia, myeloproliferative syndrome, or carcinoma is not possible. Published reports of leukemia in patients who have received oral or IV melphalan (and other alkylating drugs) suggest that the risk of leukemogenesis increases with chronicity of treatment and with cumulative dose [see HEPZATO USPI Nonclinical Toxicology (12.1)].
Embryo-Fetal Toxicity
Based on animal studies and its mechanism of action, melphalan can cause fetal harm when administered to a pregnant woman. Melphalan is genotoxic, targets actively dividing cells, and was embryolethal and teratogenic in rats. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with melphalan and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HEPZATO and for 3 months after the last dose [see HEPZATO USPI Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
Infertility
Melphalan-based chemotherapy regimens have been reported to cause suppression of ovarian function in premenopausal women, resulting in persistent amenorrhea in approximately 9% of patients. Reversible or irreversible testicular suppression has also been reported [see HEPZATO USPI Use in Specific Populations (8.3)].
(h) LOCATION OF PROCEDURE
The procedure must be performed in an appropriately equipped interventional radiology suite with fluoroscopy or an operating room designed and equipped similarly. Resuscitation personnel, equipment, and medications must be immediately available.
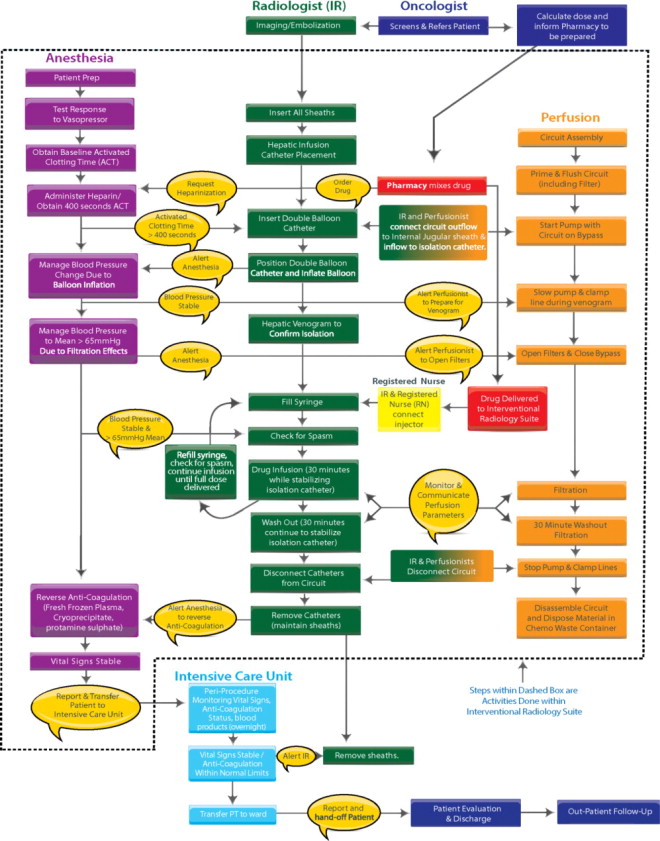
| (i) Percutaneous Hepatic Perfusion (PHP) PROCEDURE TEAM | |
| PHP Procedure team members are the Interventional Radiologist, the Perfusionist and the Anesthesiologist. | |
|
|
|
The PHP procedure team (IR, PF, AN) is required to complete the Risk Evaluation and Mitigation Strategy (REMS) training. Refer to Procedure Flowchart (Figure 35) which provides an overview of the procedure and how the PHP procedure team and their tasks work together. All REMS materials are available at www.HEPZATOKITREMS.com or by calling the REMS Coordinating Center at 1-833-632-0457.
To facilitate use of these instructions, the procedural sections include Healthcare User Identifiers to assist each user in identifying procedural steps applicable to them.
| (j) OTHER CLINICAL TEAM MEMBERS | |
| Other clinical team members include the medical/surgical oncologist, pharmacist, chemotherapy healthcare professional and intensivist. | |
|
|
|
|
|
|
|
|
|
|
| A qualified intensivist, or appropriately qualified critical care specialist, responsible for providing medical management (reversing coagulopathy and blood product support) of the patient in the immediate post-procedure period during which the patient is in the intensive care unit or step-down unit. |
PROCEDURE
(k) PREPARATION: PRIOR TO TREATMENT







All medications and supportive measures must be determined and administered in accordance with each institution's policies, guidelines, procedures, and the HEPZATO KIT prescribing information.
Before starting the procedure, confirm that all components of the HEPZATO KIT are available for assembly. Note: Certain components are not supplied by Delcath. Verify that the Medtronic pump is functioning properly (see pump operating manual for instructions on proper functionality).
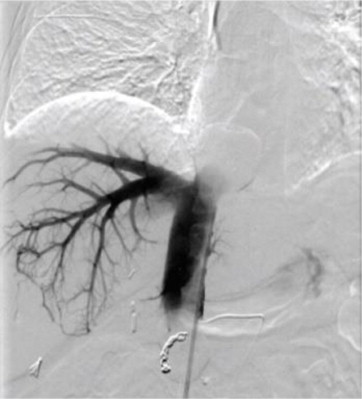
Hepatic Vascular Mapping - Angiography and Embolization
To deliver HEPZATO to the whole liver and avoid inadvertent infusion of HEPZATO into the gastrointestinal or visceral branches, conduct a thorough hepatic artery angiogram and investigation of variant hepatic and gastric artery anatomy. In addition, embolization of certain branches supplying the gastro-intestinal tract may be necessary.
| WARNING
If the perfusion of melphalan cannot be isolated from the systemic circulation, stop the drug infusion immediately. |
- Evaluate the portal vein for patency with late imaging during celiac and superior mesenteric arteriography. The presence of portal venous hypertension is a contraindication for treatment with HEPZATO via the HEPZATO KIT.
- Completely examine the arterial supply to the liver and assess and understand its impact on chemotherapy infusion. Use of a selective micro-catheter may facilitate both embolization and subsequent drug infusion.
- Assess liver blood supply and formulate a strategy for catheter placement to ensure drug infusion to the entire liver. If the risk assessment is unfavorable or the anatomic variation is too complex to allow whole liver or sequential lobar catheterization for safe delivery of melphalan, the procedure must not be performed.
- Depending on vascular anatomy, a sequential lobar approach may be the best administration option which requires splitting the HEPZATO dose. This will require repositioning of the catheter during the procedure.
- A whole liver infusion (dosing) approach will likely require embolization of the gastroduodenal artery but depends on its origin relative to the side branches of the distal proper hepatic artery. If the infusion catheter tip can be placed sufficiently distally to avoid retrograde reflux into the gastroduodenal artery, then the latter may not need to be embolized.
Double Catheter Balloon Sizing
- The double catheter balloon has two sizes representing the inter-balloon spacing distance. Spacing is based on hepatic venous anatomy and avoidance of renal vein occlusion. An inter balloon sizing should assure full isolation of all hepatic veins without occluding renal veins. Review computed tomography or magnetic resonance imaging to assess venous anatomy. Select KIT (balloon spacing) based on patient anatomy.
Coagulation Studies
- Perform coagulation studies pre-, peri- and post-procedure then repeat until normalized. Parameters tested must include:
- -
- Partial Thromboplastin Time
- -
- Prothrombin Time / International Normalized Ratio
Blood Products
Type and cross-match depending on institutional guidelines for:
- Red blood cells
- Fresh Frozen Plasma
- Platelets
- Cryoprecipitate
Hydration
- Pre procedural hydration is required if patient is determined to be hypovolemic. Do not over hydrate the patient pre-, peri- or post procedure. Overhydration has been associated with procedural and post-procedural complications.
- Procedural hydration is required since 700 mL or more of blood will be displaced into the extracorporeal circulation. A mixture of colloids and crystalloids are used to replace this volume and steadily maintain mean arterial pressure above 60 mmHg during the procedure. Typically, total procedural fluid requirements range between 1,500 and 3,000 mL.
- A Foley catheter is recommended to closely monitor fluid balance during hydration. If the patient is stable, remove within 24 hours after the procedure.
Allopurinol
- As a prophylaxis for electrolyte abnormalities, patients with more than 25% replacement of normal liver parenchyma with tumor are to be given allopurinol 300 mg/day orally beginning two (2) to three (3) days prior to percutaneous hepatic perfusion (PHP) with the Hepatic Delivery System and continuing two (2) to three (3) days following procedure.
Proton Pump Inhibitors
- Administer prophylactic proton pump inhibitors (for example: omeprazole, one 20 mg delayed release capsule by mouth) the day before and the morning of the procedure.
Anticoagulation
- The patient will be systemically anticoagulated with heparin during the procedure. Systemic anticoagulation is required to reduce the risk of thrombus formation including formation of thrombi that may impede free extracorporeal flow and filtration. Activated clotting time must be closely monitored to ensure adequate anticoagulation.
- -
- Obtain the baseline activated clotting time value.
- -
- Administer heparin to the patient only AFTER placement of the 18F (femoral vein), 10F (jugular vein), and 5F (femoral artery) sheaths. Ultrasound guidance and single anterior wall puncture technique are recommended during sheath placement to avoid bleeding complications.
- -
- The patient must be fully heparinized prior to the insertion of the Double Balloon Catheter into the inferior vena cava. Begin with an initial intravenous bolus of heparin at 300 units/kg, dose adjusted to achieve activated clotting time.
- -
- A minimum activated clotting time (ACT) of 400 seconds is necessary with a recommended ACT value > 450 seconds
- -
- Evaluate activated clotting time frequently (approximately every 5 minutes) until adequate anti-coagulation is established (ACT > 400 seconds).
- -
- DO NOT insert double balloon catheter into the patient Until ACT values are > 400 seconds.
- -
- Maintain activated clotting time at > 400 seconds throughout the procedure, assessing ACT values every 15 – 30 minutes depending on the patient's response and administering intravenous heparin as needed.
Anesthetic Management
- Treatment must be administered with patients being monitored and under general anesthesia. Emergency resuscitation equipment must be available during the procedure.
Blood Pressure Control
- Expect significant procedure related decrease of blood pressure when the balloons occlude blood return from the inferior vena cava (decreased cardiac inflow) and when the filters are brought into the extracorporeal bypass circuit. The reasons for filter-related hypotension are multifactorial, but hypersensitivity to non-physiological surfaces (inflammatory response), significant reduction in venous return and preload, and possible removal of catecholamines by the filters may play a role. To aid blood pressure maintenance for extracorporeal bypass, the following actions are recommended per institutional practice:
- -
- Pre-operative hydration and intra-procedural administration of colloids and crystalloids.
- -
- Vasopressor use in accordance with institutional practices to elevate mean arterial pressure to a target >65 mmHg
- Blood pressure must be constantly monitored throughout the procedure and maintained at levels required for adequate perfusion of critical end-organs (i.e., >65 mmHg).
Drug Preparation and Delivery Planning
- Prior to set up, provide pre-notification to the hospital pharmacy to be ready to reconstitute HEPZATO for injection.
- HEPZATO reconstitution and delivery should be coordinated and timed so that the start of the infusion of the melphalan is within thirty minutes of preparation. Drug administration should be completed within 60 minutes of the start of preparation.
- The filters should not be brought online until HEPZATO is in the procedure room. This minimizes filtration duration and associated complications.
(l) PREPARING AND PRIMING THE EXTRACORPOREAL HEMOFILTRATION CIRCUIT (EFC) 
CAUTION: Adherence to strict sterile procedures is always mandatory
-
Assemble EFC
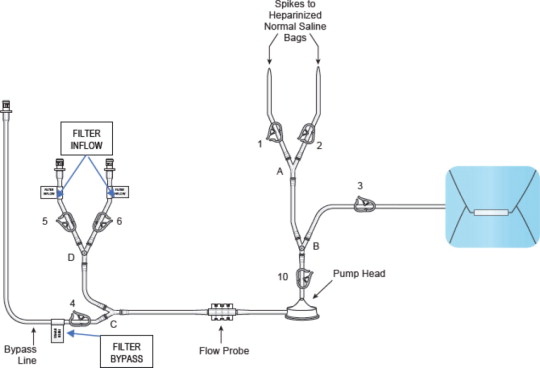
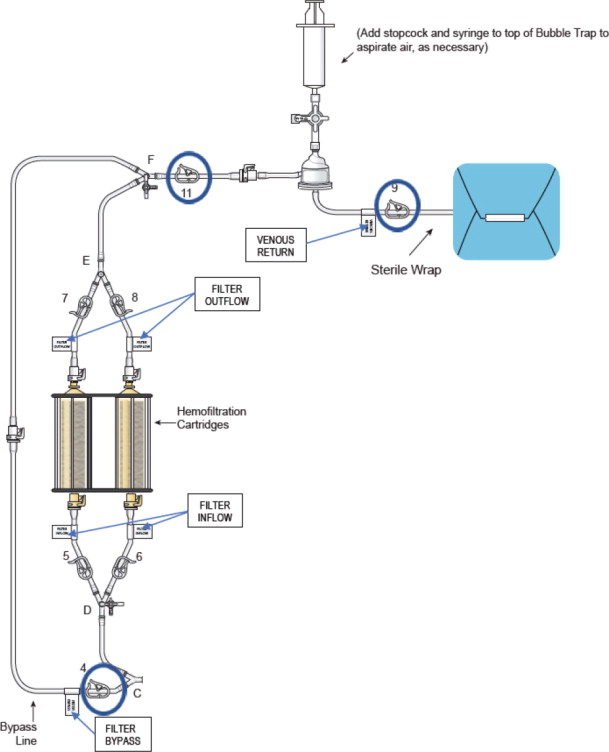
See Figure 1 (Assembled System) for reference to a completely assembled circuit.
- (a)
- Utilizing strict aseptic technique, heparinize nine (9) liters of 0.9% Sodium Chloride Injection (normal saline) by adding 2,000-5,000 units of heparin per liter.
- (b)
- Remove Hemofiltration Dual Filter Cartridge from sterile pouch.
- (c)
- Attach the filter to the intravenous pole using the built-in pole mount clamp, see Figure 3.
Figure 3: Filter mount
- (d)
- Refer to “THIS END UP” label on filter faceplate, see Figure 4.
Figure 4: Filter Orientation
- (e)
- Open the circuit tray and remove the components that are in the pouches. Set aside for later assembly.
- (f)
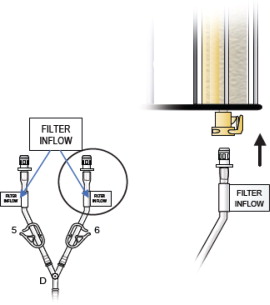
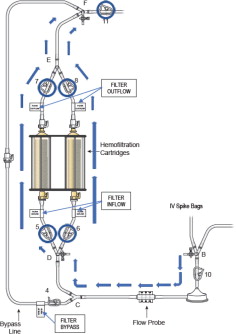
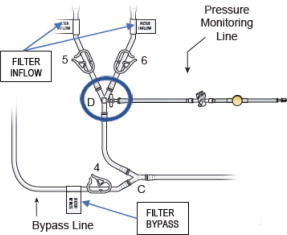
- Remove the “Filter Inflow Assembly” section from the circuit tray, see Figure 5.
- i.
- Place pump head on the pump drive motor
- ii.
- Insert flow probe into flow transducer
Figure 5: Filter Inflow Assembly
- (g)
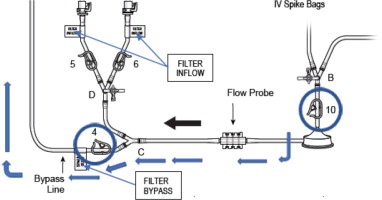
- Connect the two Inflow lines (labeled and identified as in Figure 6) to the cartridge inlet connectors located on the bottom of the dual filter. To complete the assembly, push each of the quick connector couplings together, as shown in Figure 6 (male to female) until an audible “click” is heard to verify connection (push and click).
Figure 6: Filter Inflow Assembly Connection
- (h)
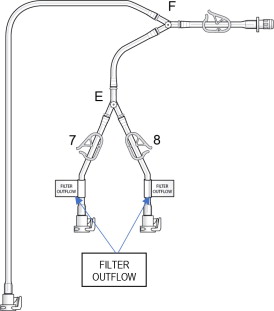
- Remove the “Filter Outflow Assembly” section from its sterile pouch, see Figure 7.
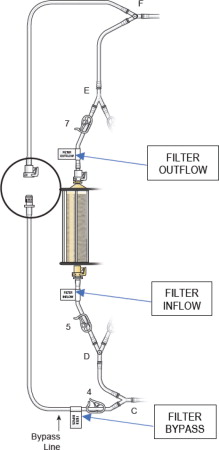
Figure 7: Filter Outflow Assembly Connection
- (i)
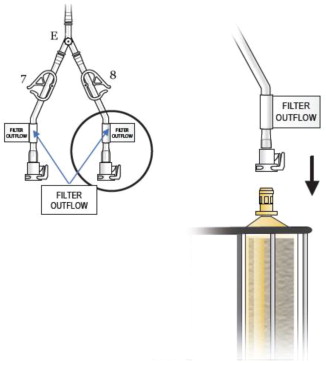
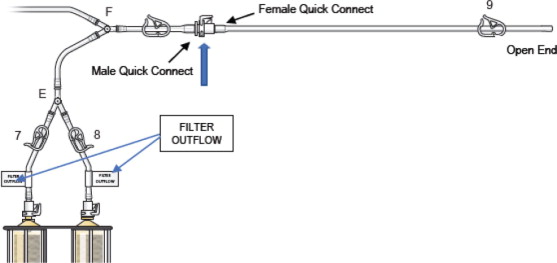
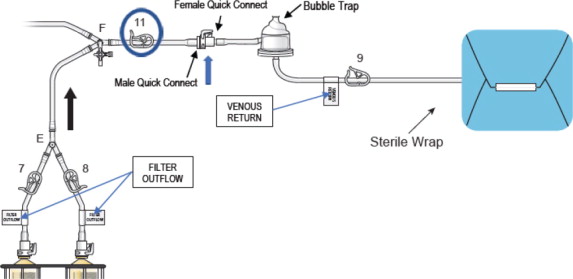
- Connect the two outflow lines to the filter cartridge outlet connectors located on the top of the dual filter using the quick connector couplings (push and click). Identify assembly labels as in Figure 8.
Figure 8: Filter Outflow Line Connection
- (j)
- Assemble the two ends of the bypass line by pushing the quick connectors together (push and click), see Figure 9 (black circle).
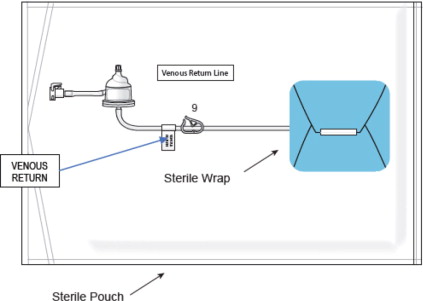
Figure 9: Bypass Line Assembly
- (k)
- Remove the “Prime/Flush Line” from its sterile pouch
- i.
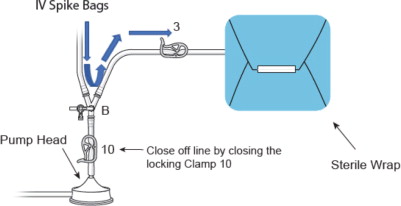
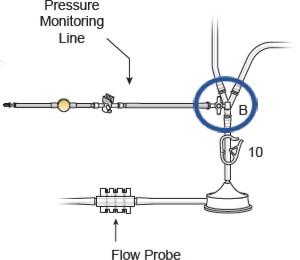
- Attach the female quick connect on the prime/flush line to the male quick connect coupling of the filter outflow assembly located distal to “Y”-connector at F (Blue Arrow), as shown in Figure 10
- ii.
- Place the open end of the “Prime/Flush Line” into the basin for collecting the flushed effluent during filter hydration
Figure 10: Prime Flush Line Assembly Connection
- (l)
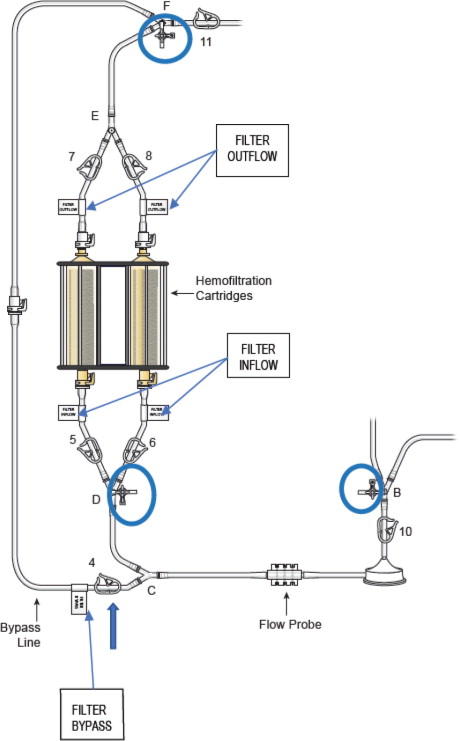
- Attach the supplied stopcocks as outlined in Figure 11 identified by the blue circles to the:
- i.
- “B” (pre-pump head pressure/suction)
- ii.
- “D” (pre-filter pressure)
- iii.
- “F” (outlet) Y-connector ports
- iv.
- Check that all stopcocks are in the closed position (lever closed to the perfusion circuit)
- v.
- Verify “Bypass Line” clamp 4 is open (Blue Arrow)
Figure 11: Stopcock Attachment Locations
- (m)
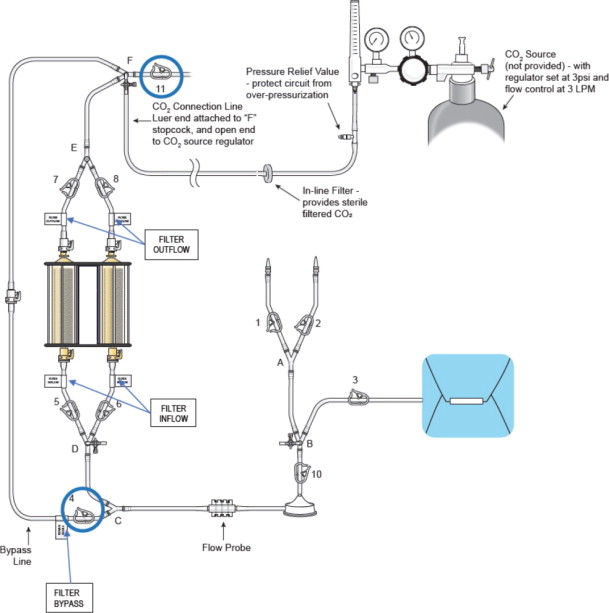
- System CO2 Prime (see Figure 12):
- i.
- Close outlet clamp (11). Note that clamps 1-10 are open
- ii.
- Attach the CO2 connection line to the stopcock “F” and open the stopcock
- iii.
- Connect the open end of the CO2 connection line to the CO2 source
- iv.
- Set the CO2 source regulator to 3psi (approximately 3 liters per minute (LPM))
- v.
- Start the CO2 gas flow and allow the CO2 to flow through the extracorporeal hemofiltration circuit. Note that CO2 flow is retrograde through the tubing set exiting at IV spikes (clamps 1 &2) and blue sterile pouch (clamp 3)
- vi.
- Adjust CO2 regulator to maintain 3psi (as necessary). Verify CO2 flow through the circuit
- vii.
- Close bypass clamp (4) after approximately 1 minute to ensure flow through the hemofiltration cartridges
- viii.
- Allow CO2 to flow through the cartridges (after closing clamp 4) for at least 5 minutes but preferably 15 minutes
- ix.
- After the desired CO2 prime duration, close the following clamps to pressurize the system with CO2 and prevent room air from entering:
- a.
- Saline spike clamps (1, 2)
- b.
- Double balloon catheter line clamp (3)
- c.
- Filter inlet clamps (5, 6)
- d.
- Filter outlet clamps (7, 8)
- x.
- Stop the CO2 flow and close the stopcock “F”
- xi.
- Disconnect the CO2 connection line and discard
Figure 12: CO2 Priming Connections
- (n)
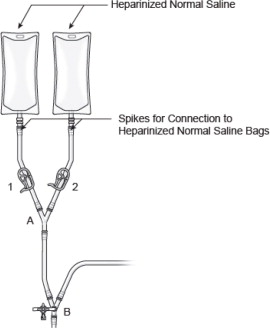
- Hang two bags of the heparinized sterile normal saline and connect to circuit by using the spikes, as shown in Figure 13, to allow for gravity priming of circuit components. CAUTION: Use strict aseptic technique while spiking the heparinized normal saline bags.
Figure 13: Connecting Heparinized Saline Bags
-
Prime Double Balloon (Isolation Aspiration) Catheter Line
- (a)
- Close pre-pump clamp (10).
- (b)
- Confirm that stopcock “B” is in the closed position (the lever is pointed toward the circuit tubing to the right).
- (c)
- Open double balloon catheter line clamp (3).
- (d)
- Open saline line (clamp 1 or 2), to allow heparinized normal saline to prime line only up to clamp 3, see Figure 14; blue arrows demonstrating flow direction. Do not allow excess heparinized normal saline to fill sterile wrap.
- (e)
- Close clamp 3.
Figure 14: Prime Double Balloon Isolation Aspiration Catheter Line
- Prime Bypass Line and Pump Head (see Figure 15):
-
Prime and Flush Dual Filter Cartridge (see Figure 16 location of clamps and prime flow direction)
CAUTION: Do NOT allow heparinized normal saline bags to run dry or air will enter the system.
- (m)
- Inspect that stopcock “F” is in the closed position (the lever is pointed upward).
- (n)
- Open filter inlet clamps (5, 6).
- (o)
- Open filter outlet clamps (7, 8).
- (p)
- Open circuit outlet clamp (11).
- (q)
- Adjust the flow of heparinized normal saline into the filter to a rate of approx. 0.5 liters per minute. Note: Hemostats (forceps) are required to adjust flow rate if using gravity.
Figure 16: Priming Filter Cartridges
- (r)
- Note that filter cartridges will have a mottled appearance indicating the presence of gas bubbles.
- (s)
- Allow heparinized normal saline to flow through the filters and out the “Prime/Flush Line” for approximately 6-10 minutes or until the filter appears gas free (solid black).
- (t)
- Once all gas appears to have been displaced, gently roll the cartridges between palm of hands to encourage trapped gas bubbles to rise. CAUTION: Do not use excessive force when rolling the plastic housing.
- (u)
- Inspect the entire cartridge for trapped gas by turning the cartridge within housing to visualize the entire filter. If there are gas bubbles gently roll the cartridges to free the trapped gas.
- (v)
- When Filter Cartridges are gas free, flush with an additional six (6) liters of heparinized normal saline (3 L/cartridge).
- (w)
- Close filter clamps 5, 6, 7, 8 and outlet clamp 11.
-
Prime Venous Return Line and Bubble Trap
CAUTION: Do NOT install the Return Line with built-in Bubble Trap until flushing is complete
- (a)
- Disconnect and dispose of the “Prime/Flush Line”, by pressing in the latch located on the female quick connect coupling and pulling it a part (refer to Figure 10).
- (b)
- Open the Venous Return sterile pouch (see Figure 17) and remove the venous return line and built-in bubble trap.
Figure 17: Venous Return Line with Built-In Bubble Trap
- (c)
- Attach the female quick connect of the venous return line to the male quick connector (push and click) located (blue arrow) by outlet clamp (11), as shown in Figure 18. Position the bubble trap in the bubble trap holder higher than filter cartridges.
Figure 18: Connecting Venous Return Line with Built-In Bubble Trap
- (d)
- Attach stopcock to bubble trap and use syringe to aspirate air, as necessary, see Figure 19.
- (e)
- Prime venous return line and bubble trap by opening clamps 4, 11 and 9
- (f)
- Prime up to clamp 9. Do not allow saline to enter blue sterile pack.
- (g)
- Close clamp 9 once venous return line and bubble trap are primed up to clamp 9.
Figure 19: Priming Venous Return Line with Built-In Bubble Trap
-
Install Pressure Monitoring Lines
- (a)
- Attach pre-pump (to measure negative pressure – pump suction) pressure monitoring line to stopcock “B” and prime, see Figure 20.
Figure 20: Attach and Prime Pre-Pump Pressure Line
- (b)
- Attach pre-filter (to measure positive pressure – pre-filter) pressure monitoring line to stopcock “D” and prime, see Figure 21.
Figure 21: Attach and Prime Pre-Filter (Post-Pump) Pressure Line
- (c)
- Attach the pressure monitoring lines to the P1 and P2 ports on the rear of the Medtronic Bio-Console 560 Speed Controller System.
- (d)
- Zero the pressure transducers (refer to Medtronic Bio-Console 560 System Manual for details).
- (e)
- Coiled pressure monitoring lines are included for use with DLP Pressure Display Boxes, as necessary.
-
Pressure Test Circuit
- (a)
- Pressure test circuit by slowly ramping up the pump head speed (RPM) until a pressure reading of 300 mmHg is achieved on the pressure transducer attached to the line on Y-connector “D” (pre-filter).
- (b)
- Visually inspect all connections and cartridges to ensure no leaks are present.
CAUTION: If leak is noted, ensure connections are secure before proceeding.
- (c)
- Turn off pump and close cartridge inlet (5, 6) and outlet (7, 8) clamps. Ensure bypass line clamp (4) is open.
- (d)
- System is now primed, hydrated, de-bubbled, and ready for use.
- (e)
- Ensure there are two (2) liters of normal saline available for later use.
WARNING
Ensure that all air is purged from the system prior to use in order to avoid an air embolism(x) PLACING THE CATHETERS

-
Insertion of the 10F Venous Return Sheath
- (a)
- Attach the stopcock to the sheath side port tube.
- (b)
- Using standard Seldinger technique (recommended ultrasound guidance), insert the venous return sheath, into the internal jugular vein (preferably the right side internal jugular vein, see Figure 22). Use of sonographic guidance and a single anterior wall puncture of the vein is recommended to avoid inadvertent carotid artery puncture. If carotid artery puncture occurs, the procedure must be aborted and postponed to a later date, due to hemorrhage risk with high level of anticoagulation.
- (c)
- Flush the sheath with sterile heparinized normal saline.
- (d)
- Close the stopcock.
Figure 22: Placing Venous Return Sheath into Internal Jugular Vein
-
Insertion of the 5F Femoral Arterial Sheath
- (a)
- Using Seldinger puncture technique and standard fluoroscopic and arteriographic techniques, place the 5F Introducer Sheath into the femoral artery, see Figure 23.
- (b)
- Recommend sonographic guidance and a single anterior wall puncture of the femoral artery over the femoral head to assure compressibility of the artery when the sheath is removed. If a supra-inguinal puncture is made, the procedure must be aborted and postponed to a later date.
Figure 23: Placing Femoral Artery Sheath
-
Insertion of the 18F Venous Sheath
- (a)
- Using Seldinger technique and standard fluoroscopic and angiographic techniques, place the 18F introducer sheath into the femoral vein after serial dilation with 9F and 13F dilators. The venous sheath may be placed ipsilateral or contralateral to the 5F femoral arterial sheath placement, see Figure 24.
- (b)
- Flush the sheath with sterile heparinized normal saline.
- (c)
- Recommend use of sonographic guidance and a single anterior wall puncture of the femoral vein over the femoral head to assure compressibility of the vein when the sheath is removed. If a supra-inguinal puncture is inadvertently made, the procedure must be aborted and postponed to a later date.
Figure 24: Placing Femoral Venous Sheath
-
Insertion of 5F Infusion Catheter
- (a)
- Introduce the 5F Infusion catheter through the sheath and manipulate it over a guidewire into the proper hepatic artery, see Figure 25. At the discretion of the Interventional Radiologist, a microcatheter may be coaxially introduced through the 5F catheter for selective catheter tip placement for drug infusion. If a microcatheter is used, attach a rotating hemostatic valve (Touhy-Borst type) to the 5F catheter and insert the microcatheter into the 5F catheter through the valve. Three microcatheters have been qualified by Delcath for use with the HEPZATO for Injection/Hepatic Delivery System. Select one of the three microcatheters qualified (see Description of System Components (pg. 5).
WARNING
To deliver melphalan to the whole liver and avoid inadvertent infusion of melphalan into the gastrointestinal or visceral branches, conduct a thorough hepatic artery angiogram and investigation of variant hepatic and gastric artery anatomy. In addition, embolization of certain branches supplying the gastro-intestinal tract may be necessary.
The catheter must be positioned as described below so that drug Is infused ONLY into the liver. Perfusion of drug into any other abdominal organ or gastrointestinal branches must be avoided as this may result in serious injury or death. - (b)
- Position the infusion catheter (5F catheter or microcatheter) in the hepatic artery for intra hepatic HEPZATO administration and to exclude HEPZATO delivery into extrahepatic gastric side branches either directly or in the case of reflux. Affix the 5F catheter to the skin at the groin.
- (c)
- Connect the infusion catheter (5F catheter or microcatheter) to the drug delivery system (see step 18) and maintain catheter patency by hospital catheter infusion protocols (e.g., infuse heparinized normal saline: The concentration of heparin should be 1,000 units per 500 mL of normal saline).
Figure 25: Insertion of Hepatic Artery Infusion Catheter
(y) ESTABLISHING ANTICOAGULATION & PLACING DOUBLE BALLOON CATHETER



-
Anticoagulation
- (a)
- DO NOT place the double balloon catheter until the patient is fully anticoagulated.
- (b)
- DO NOT administer heparin until the placements of all vascular sheaths.
- (c)
- Obtain the baseline activated clotting time value.
- (d)
- Administer heparin.
- (e)
- Administer an initial intravenous bolus of 300 units/kg of heparin. Heparin dose should be adjusted to achieve a minimum activated clotting time of 400 seconds prior to initiation of veno-venous bypass and balloon inflation.
- (f)
- Evaluate activated clotting time frequently (approximately every 5 minutes) until adequate anti-coagulation is established (activated clotting time > 400 seconds). Maintain activated clotting time at > 400 seconds (preferably > 450 seconds) throughout the procedure, obtaining ACT values every 15-30 minutes depending on the patient's response, and administering intravenous heparin as needed.
WARNING
The start of the intra-arterial infusion of the drug solution must be within 30 minutes of its preparation.NOTE: TIMING OF CHEMOTHERAPEUTIC AGENT DELIVERY
Time the request for delivery of the chemotherapeutic agent (HEPZATO) so that the start of the intra-arterial infusion of the drug solution is within thirty minutes of its preparation. Since preparation and delivery times vary, depending upon local practices, the timing of the request is critical and should be pre-arranged with the pharmacist. Ideally, the time to request chemotherapeutic agent from the pharmacy would be when the patient is heparinized just prior to placing the Double Balloon Catheter.
-
Insertion of the Double Balloon Catheter
- (a)
- Flush the Double Balloon catheter with heparinized normal saline.
- (b)
- Introduce the Double Balloon catheter through the 18F sheath. Under fluoroscopic guidance advance it over a guidewire into the inferior vena cava and position the catheter tip at the level of diaphragmatic hiatus. Do NOT expand the balloons.
- (c)
- Upon successful placement, remove guidewire and create a heparin lock within the “OTW” lumen to maintain patency.
(z) CONNECTING CATHETERS TO EXTRACORPOREAL HEMOFILTRATION CIRCUIT


-
Connection of Catheter to Extracorporeal Hemofiltration Circuit
- (a)
- Remove sterile wrap from extracorporeal hemofiltration circuit double balloon catheter line while maintaining sterility and transfer sterile end to interventional radiologist.
- (b)
- Open saline line (clamp 1 or 2) clamp and clamp 3 to allow for a “wet connection” of the extracorporeal hemofiltration circuit to the double balloon catheter large drainage lumen with a quick connect fitting, see Figure 26. After connection is made, close saline line clamp (clamp 1 or 2, same ones opened previously). Ensure that all air is removed from the double balloon catheter.
Figure 26: Connecting Double Balloon Catheter to Circuit
- (c)
- Remove the sterile wrap from the extracorporeal hemofiltration circuit venous return line while maintaining sterility and transfer sterile end to the interventional radiologist and flush normal saline to fill the line.
- (d)
- Connect the extracorporeal hemofiltration circuit venous return line to the stopcock of the 10F venous return sheath placed in the jugular vein (Venous Return Sheath tubing has a red clamp), flush normal saline through the line. When all air is removed, and line is completely filled with normal saline turn stopcock to close the side port. Ensure the stopcock (at the venous return line to sheath connection) is fully open to minimize back pressure and maximize flow through the stopcock. (“OFF” handle of stopcock turned 90° to flow path.)
-
Establishing Hemofiltration Circulation
- (a)
- Confirm filter inlet and outlet clamps are CLOSED (5, 6, 7 and 8) (red circles), per Figure 27.
- (b)
- Open clamp 9 (blue circles).
- (c)
- Confirm that clamps 3, 4, 10 and 11 are open (blue circles)
- (d)
- Start pump and slowly increase RPM control to achieve a maximum allowable flow rate which does not cause flow induced vibration or exceed the 0.80 L/min flow rate or −250 mmHg pre-pump pressure.
- Flow rates of approximately 0.40 to 0.60 liters/minute are typical: 0.40 L/min is the minimum allowable and 0.80 L/min is the maximum allowable flow rate for this system.
- In-line pressure transducers should be used to monitor pressures:
- Pre pump pressure (suction side) should not be more negative than −250 mmHg, as lower pressures indicate possible catheter collapse or kink.
- Pre-cartridge pressures (pre-filter) should not exceed 200 mmHg, as higher pressures indicate increasing filter resistance potentially due to thrombus or a kinked return line. Check filters to assure free flow and return line for kinks.
- (e)
- The extracorporeal hemofiltration circuit is now established. Venous blood is aspirated from the central lumen through the fenestrations in the Double Balloon catheter. This blood flows through the Double Balloon catheter to the pump, through the bypass line, and returns to the patient through the venous return sheath.
CAUTION: Continuously monitor any perfusion related events including:
- Blood flowrate as displayed by the Medtronic Bio-Console
- Systolic, diastolic and mean arterial blood pressure
- Heart rate and vital signs
- Activated clotting times
- Bubble-trap for entrapped air
- Leaks from any part of the circuit
Figure 27: Establishing Hemofiltration Circulation
(aa) ISOLATING THE INFERIOR VENA CAVA



-
Expansion of Balloons
WARNING
There is an anticipated significant decrease of blood pressure following the initial occlusion of the inferior vena cava by the balloons. It is critical to maintain mean blood pressure above 65 mmHgVasoactive Agents Response Testing: Prior to expansion of either balloon (occlusion of inferior vena cava), administer vasoactive agent to assess patient responsiveness to this agent. It is recommended to use an infusion pump to quantify vasopressor doses during each procedural period. After balloons expansion, assess patient blood pressure for two (2) to five (5) minutes before proceeding. Significant decreases in blood pressure will occur within two (2) to five (5) minutes.
Continue to administer vasoactive agents to maintain mean blood pressures above 65 mmHg. Vasopressor agents are typically not required after the conclusion of the procedure.
- (a)
- Perfusionist must carefully monitor the flow rate during the balloon expansion.
WARNING
Do NOT Over Expand the Balloons. Over Expansion of the Balloons Could Cause the Balloons to Burst which could result in Life-Threatening Injury. - (b)
- Maximum balloon expansion volumes:
- Cephalad Balloon:
- Caudal Balloon:
38 mL of dilute contrast medium
38 mL of dilute contrast medium - (c)
- Under fluoroscopy, partially expand the cephalad balloon with approximately 15 - 25 mL of dilute contrast media (e.g., 35% dilution) within the right atrium (the balloon will have a rounded appearance).
- (d)
- With the caudal balloon collapsed, slowly retract the Double Balloon catheter until the cephalad balloon is at the junction of the right atrium and inferior vena cava. If needed, further expand the cephalad balloon until indentation of the diaphragmatic hiatus is visible at the inferior margin (the balloon will acquire an acorn shaped appearance, see Figure 28). Do not expand balloons beyond required volume to achieve an adequate seal. Never advance or retract the Double Balloon catheter when both balloons are expanded. If resistance is met during manipulation, determine the cause of the resistance before proceeding.
Figure 28: Expansion and Placement of Cephalad Balloon
- (e)
- Under fluoroscopy, expand the caudal balloon with dilute contrast medium until the lateral edges of the expanded balloon start to become effaced by the inferior vena cava wall.
WARNING
Never stop blood flow through the extracorporeal hemofiltration circuit for more than 30 seconds. - (f)
- With balloons expanded, perform a limited (retro-hepatic) inferior vena cavagram (using digital subtraction angiography technique) through the fenestrations. Prior to injection of contrast medium, reduce the pump speed to 1,000 RPM and clamp off the circuit. Inject iodinated contrast medium through the CONTRAST port to confirm that the catheter properly isolates hepatic venous flow between the balloons. The cephalad balloon must occlude the inferior vena cava just above the highest (closest to right atrium) hepatic vein, and the caudal balloon must occlude the inferior vena cava just below the lowest hepatic vein (above the renal veins) as shown in the radiographic image in Figure 29.
- (g)
- Re-establish flow through the extracorporeal hemofiltration circuit by unclamping the circuit and returning pump RPM to deliver previous flow rate.
Figure 29: Confirming Hepatic Venous Isolation
WARNING
Never Adjust the Position of the Double Balloon Catheter Unless Both Balloons are Fully Collapsed - (h)
- If the Double Balloon catheter is not in the proper position, collapse both balloons (caudal balloon first) and then reposition the catheter, while maintaining flow in the extracorporeal hemofiltration circuit.
- (i)
- Once satisfactory position is attained (i.e., the isolated segment is well sealed), gently hold the proximal end of the Double Balloon catheter to prevent upward migration of the catheter into the right atrium. The catheter must be held due to forces that will push the balloon upwards towards the right atrium, and its position checked for the duration of the procedure (approximately 60 minutes).
CAUTION: Check Double Balloon catheter balloon positions fluoroscopically every four (4) to five (5) minutes during drug administration and filtration to ensure continued hepatic venous isolation.
(bb)
(cc) BRINGING HEMOFILTRATION CARTRIDGES ONLINE


-
Bringing Hemofiltration Cartridges online
- (a)
- HEPZATO preparation should be in the procedure room prior to bringing filters online. This reduces filter related complications and occupation of melphalan binding sites by blood components.
- (b)
- Continuously monitor and check the patient's blood pressure as required (see “Blood Pressure Control”).
- (c)
- Typically, each filter is brought online sequentially while leaving the bypass line open.
- (d)
- Bring the left cartridge online by opening clamps 5 and 7 (see blue circles) allowing blood to displace the heparinized normal saline into the patient.
- (e)
- Observe and manage blood pressure.
- (f)
- After the heparinized normal saline in the left cartridge and its lines is fully replaced with blood, wait approximately 30 seconds and open right cartridge clamps 6 and 8 (see green circles), while keeping the bypass line open.
- (g)
- Once the heparinized normal saline in the right cartridge and its lines is fully replaced with blood, wait approximately 30 seconds managing blood pressure.
- (h)
- Once blood pressure is stable close the bypass line by securely closing clamp 4 (red circle), see Figure 30. Add a reusable tube clamp as a redundant bypass closure mechanism high on the bypass line in clear view of the team.
- (i)
- Manage and stabilize blood pressure.
Figure 30: Bringing Hemofiltration Cartridges online
WARNING
Close bypass line prior to infusion of drug. Never reopen bypass line(dd) SETUP DRUG DELIVERY SYSTEM AND START EXTRACORPOREAL FILTRATION


-
Drug Administration and Extracorporeal Filtration
HEPZATO is prepared by Pharmacy per physician's prescription. Reconstitute each melphalan vial with 10 mL of supplied diluent.
- For doses up to 110 mg, dilute reconstituted HEPZATO in 250 mL 0.9% Sodium Chloride Injection (use one (1) 250 mL bag provided).
- For doses between 111 mg and 220 mg, divide the total dose equally into 2 and dilute each in 250 mL 0.9% Sodium Chloride Injection (total volume of 500ml using the two (2) 250 mL bags provided).
Multiple injection cycles of the diluted HEPZATO solution will be required (see delivery parameters described below).
WARNING
The start of the intra-arterial infusion of the drug solution must be within 30 minutes of its preparation in the pharmacy.
The duration of time between filters brought online and start of HEPZATO administration should be minimized on the order of < 5 minutes- To deliver the drug solution, divide total volume into volumes within the capacity of the injector syringe.
- Deliver drug solution at a flow rate of 25mL/min. Refill syringe after each injection cycle.
- Conduct spasm check of the hepatic arteries and confirm balloon position and expansion against vena cava walls by fluoroscopy while refilling syringe.
- Continue infusion until all drug is delivered within 30 minutes. Below are the delivery parameters:
- -
- Total volume of 500 mL – 5 infusions of 100 mL at 25mL/min
- -
- Total volume of 540 mL – 5 infusions of 100 mL + infusion of 40 mL at 25mL/min
- -
- Total volume of 250 mL – 2 infusions of 100 mL + infusion of 50 mL at 25 mL/min
- -
- Total volume of 270 mL – 2 infusions of 100 mL + infusion of 70 mL at 25 mL/min
- (a)
- Set-up drug delivery system by installing a 150 mL syringe into the drug injector and setting the injector flow rate to 25 mL/minute and enter the injection volume determined as described above. See Figure 31 for HEPZATO administration set up.
- (b)
- Attach intravenous fluid administration sets to the other ports of the two stopcocks, as shown in Figure 31.
Figure 31: HEPZATO Administration System Setup
- (c)
- Prepare, label, and connect three (3) syringes: One (1) for contrast, one (1) for intra-arterial nitroglycerin (IANTG), and one (1) for normal saline. An example setup is depicted in Figure 32.
- i.
- Undiluted iodinated contrast agent is for checking hepatic artery spasm via CT. The contrast is injected by hand via the syringe for the arteriogram.
- ii.
- Nitroglycerin is prepared for use, as needed, to relieve hepatic artery spasm.
- iii.
- Normal saline is drawn into the saline syringe for priming and flushing the hepatic arterial infusion line during the procedure.
Figure 32: Manifold Setup of for Nitroglycerin, Contrast and Saline
Draw the following volumes and concentrations of contrast, nitroglycerin, and normal saline into the three syringes of the drug delivery set-up.
Syringe Volume and Concentration to Prepare in Syringe Contrast Use institutional practices for microcatheter contrast delivery NTG Use institutional practices Normal Saline 20 mL of 0.9% Sodium Chloride Injection - (d)
- Hang a 500 mL bag of normal saline and the chemotherapeutic agent (melphalan hydrochloride) drug solution bag on the intravenous pole and ensure the roller clamps are closed, and spike the bags, as shown in Figure 33. Visually inspect the solution for particulates. If particulates are observed, DO NOT USE.
Figure 33: HEPZATO Administration System Setup
- (e)
- Prime the drug delivery system with normal saline, fill injector syringe with approximately 120 mL of diluted drug. Ensure all air is purged from the system.
- (f)
- When the extracorporeal hemofiltration circuit is running satisfactorily and the patient is hemodynamic stable, flush the hepatic arterial infusion line with normal saline to avoid directly mixing heparin with chemotherapeutic agent (melphalan hydrochloride). Connect drug infusion line to Hepatic Artery Infusion catheter (5F Catheter or microcatheter) to complete the drug delivery circuit.
- (g)
- Perform an arteriogram to assess patency of the hepatic artery. Use undiluted iodinated contrast agent to check for hepatic artery spasm via CT. The contrast is injected by hand via the syringe for the arteriogram. In circumstances where hepatic arterial spasm is noted, administer nitroglycerin intra-arterially to alleviate the spasm. Always flush the injection line with normal saline after contrast injections.
WARNING
Assess arterial patency approximately every four (4) to five (5) minutes via contrast administration during drug infusion. Administer intra-arterial nitroglycerin if arterial spasm is noted. If spasm cannot be relieved, terminate the procedure (see ending extracorporeal circulation below).- (h)
- Initiate administration of the 1st dose of chemotherapeutic agent (melphalan hydrochloride) through the Infusion catheter.
WARNING
Immediately stop the procedure if perfusion of drug is detected outside of the isolated region and cannot be corrected. Once the infusion of chemotherapeutic agent (melphalan hydrochloride) has started, do not collapse balloons unless administration of drug has been stopped and a full washout cycle (30 minutes) has been completed.- (i)
- After the prescribed dose has been fully administered, continue extracorporeal filtration for an additional 30 minutes (washout period).
(ee) ENDING EXTRACORPOREAL CIRCULATION


-
Ending Extracorporeal Circulation
- (a)
- At the end of the 30-minute wash-out period, collapse the caudal balloon fully.
- (b)
- Then collapse the cephalad balloon fully.
- (c)
- Discontinue filtration by:
- i.
- Reducing the pump RPM to 1,000,
- ii.
- Closing clamps 3 and 9 See Figure 34 (red circles).
- iii.
- Stop flow by turning off the pump.
Figure 34: Close Circuit Inflow and Circuit Return Clamps to Discontinue Extracorporeal Circulation
(ff) CATHETER REMOVAL

-
Catheter Removal
- (a)
- When melphalan infusion is completed remove the guide catheter and infusion microcatheter from femoral artery access. The 5F arterial sheath should only be removed when coagulation status has been normalized.
- (b)
- When washout phase is completed remove Double Balloon catheter from the femoral vein access and replace with 18F obturator. Place the obturator completely into the sheath so the obturator hub bottoms out onto the sheath hub. The 18F venous sheath should only be removed when coagulation status has been normalized.
- (c)
- Close the stopcock or red clamp on the 10F venous return sheath side port and disconnect the venous return line from the sheath. Do not remove the 10F venous return sheath until coagulation status has been normalized.
- (d)
- Dispose of all components appropriately in accordance with hospital, local, state, and federal biohazard guidelines.
(gg) NORMALIZATION OF COAGULATION STATUS FOR SHEATH REMOVAL



-
Normalization of Coagulation Status for Sheath Removal
- (a)
- Administer protamine sulfate by slow intravenous infusion in a dose appropriate to the amount of heparin given and the activated clotting time.
- (b)
- Administer 10 units of cryoprecipitate and/or Fresh Frozen Plasma based on coagulation profiles to correct remaining abnormalities, if indicated, per institutional guidelines.
- (c)
- Repeat coagulation profile.
- (d)
- Correct remaining coagulopathy following institutional guidelines. The following recommendations are provided for consideration:
Coagulation Profile Action Prothrombin time greater than 2 seconds of normal Administer Fresh Frozen Plasma Partial thromboplastin time greater than 5 seconds of normal Administer protamine - (e)
- Measure blood platelet levels to determine if replacement is needed.
- (f)
- Follow institutional guidelines for administration of packed red blood cells for anemia.
- (g)
- All sheaths may be removed if the platelet count is greater than 50,000/ mm3 and after the patient's coagulation status has normalized. Compress puncture sites until adequate hemostasis is achieved.
- (h)
- Dispose of all components appropriately in accordance with hospital, local, state, and federal biohazard guidelines.
- (i)
- Carefully monitor the patient until complete recovery.
(hh) PROCEDURE FLOWCHART - FIGURE 35
(ii)
| Manufactured by: Delcath Systems, Inc. Queensbury, NY 12804 | |||
|
| |||
| Delcath is a registered trademark of Delcath Systems, Inc. HEPZATO is a registered trademark of Delcath Systems, Inc. HEPZATO KIT is a registered trademark of Delcath Systems, Inc. © 2023 Delcath Systems, Inc. All rights reserved. Medtronic's Bio-Console® , Bio-Pump, and Bio-Probe are registered trademarks of Medtronic Inc. | Recyclable
Package |
||
Principal Display Panel - Kit Label
NDC 75833-601-02
HEPZATO KIT™
(melphalan) for Injection/
Hepatic Delivery System (HDS)
REF 601002
EXP: YYYY-MM-DD
LOT: XXXXXXX
Contents:
1 - Hepatic Delivery System, 62 mm Double Balloon Catheter
1 - HEPZATO™ 5 x 50 mg/vial* (melphalan) for Injection and (2) 250 mL, 0.9% Sodium Chloride
Injection USP (secondary diluent):
WARNING: Hazardous Drug
SINGLE-DOSE VIAL
Rx Only
*HEPZATO Carton contains 5 vials of HEPZATO and 5 vials of sterile diluent:
*Each vial contains sterile, non-pyrogenic, freeze dried melphalan 50 mg, equivalent to 56 mg of
melphalan hydrochloride and 20 mg povidone.
Each vial of sterile, nonpyrogenic diluent containing 0.2 g sodium citrate, 6.0 mL propylene glycol,
0.52 mL ethanol (96%), and Water for Injection to a total of 10 mL.
Must be reconstituted with accompanying diluent and further diluted with accompanying 0.9%
Sodium Chloride Injection USP. See enclosed prescribing information for additional reconstitution
and administration instructions.
Recommended Dosage: see Prescribing Information
For intra-arterial infusion only
Store at controlled room temperature 20° to 25°C (68° – 77°F). Temperature excursions are
permitted between 15° to 30°C (59° – 86°F) [see USP Controlled Room Temperature]
Protect from light. Retain in carton.
Consult the HEPZATO KIT™ Instructions for Use prior to use.
Caution: This Double Balloon Catheter Contains Natural Rubber Latex
Which May Cause Allergic Reactions.
Delcath®
Packaged and Distributed by:
Delcath Systems, Inc.
Queensbury, NY 12804
Principal Display Panel – 50 mg Vial Label
NDC 75833-800-01
Rx Only
SINGLE-DOSE VIAL
HEPZATO ™
(melphalan) for Injection
50 mg/vial*
WARNING: Hazardous Drug
*Each vial contains sterile, non-
pyrogenic, freeze dried melphalan 50
mg, equivalent to 56 mg melphalan
hydrochloride and 20 mg povidone
For intra-arterial infusion only with
the Delcath Hepatic Delivery System
90004.B
Principal Display Panel – 10 mL Diluent Vial Label
NDC 75833-700-01
Rx Only
SINGLE-DOSE VIAL
STERILE DILUENT
for HEPZATO ™
(melphalan) for Injection
For Drug Diluent Use Only
Each vial contains 0.2 g sodium citrate, 6.0 mL
propylene glycol, 0.52 mL ethanol (96%),
and Water for Injection
Nonpyrogenic
90003.B
Principal Display Panel - 0.9 g/250 mL Container Label
0.9% Sodium Chloride
Injection USP
REF L8002
NDC 0264-7800-20
DIN 01924303
HK 22617
250 mL
EXCEL® CONTAINER
Each 100 mL contains: Sodium Chloride USP 0.9 g;
Water for Injection USP qs
pH adjusted with HCl NF
pH: 5.6 (4.5–7.0); Calc. Osmolarity:310 mOsmol/liter
Electrolytes (mEq/liter): Na+ 154; Cl– 154
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.
WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-223
LD-136-4
EXP
LOT
| HEPZATO KIT
melphalan hydrochloride injection, powder, lyophilized, for solution kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Delcath Systems, Inc. (847060225) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Delcath Systems, Inc. | 078698330 | MANUFACTURE(75833-601) | |
More about Hepzato (melphalan)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: alkylating agents