Haemonetics Anticoagulant Sodium Citrate Prescribing Information
Package insert / product label
Generic name: trisodium citrate dihydrate
Dosage form: solution
On This Page
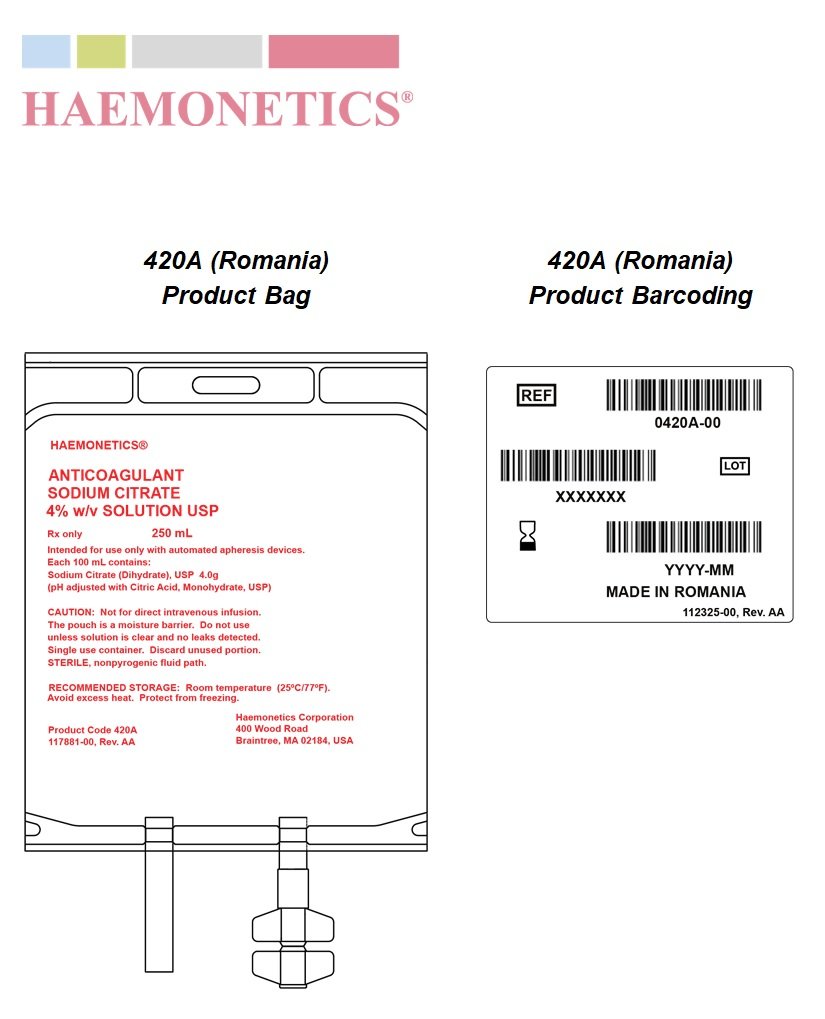
HAEMONETICS ANTICOAGULANT SODIUM CITRATE 4% w/v SOLUTION USP
Rx only 250 mL
Intended for use only with automated apheresis devices.
Each 100 mL contains: Sodium Citrate (Dihydrate), USP 4.0g
(pH adjusted with Citric Acid, Monohydrate, USP)
Warnings
Not for direct intravenous infusion. The pouch is a moisture barrier. Do not use unless solution is clear and no leaks detected. Single use container. Discard unused portion.
| HAEMONETICS ANTICOAGULANT SODIUM CITRATE
trisodium citrate dihydrate solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Haemonetics Corporation (057827420) |