Andexxa Prescribing Information
Package insert / product label
Generic name: andexanet alfa
Dosage form: injection, powder, lyophilized, for solution
Drug class: Anticoagulant reversal agents
J Code (medical billing code): J7169 (10 mg, injection)
Medically reviewed by Drugs.com. Last updated on Apr 15, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ANDEXXA® (coagulation factor Xa (recombinant), inactivated-zhzo)
Lyophilized powder for solution for intravenous injection
Initial U.S. Approval: 2018
WARNING: THROMBOEMBOLIC RISKS, ISCHEMIC RISKS, CARDIAC ARREST, AND SUDDEN DEATHS
See full prescribing information for complete boxed warning
Treatment with ANDEXXA has been associated with serious and life-threatening adverse events, including: (5.1)
- •
- Arterial and venous thromboembolic events
- •
- Ischemic events, including myocardial infarction and ischemic stroke
- •
- Cardiac arrest
- •
- Sudden deaths
Monitor for thromboembolic events and initiate anticoagulation when medically appropriate. Monitor for symptoms and signs that precede cardiac arrest and provide treatment as needed.
Indications and Usage for Andexxa
ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a recombinant modified human factor Xa (FXa) protein indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. (1)
This indication is approved under accelerated approval based on the change from baseline in anti-FXa activity in healthy volunteers. An improvement in hemostasis has not been established. Continued approval for this indication may be contingent upon the results of studies that demonstrate an improvement in hemostasis in patients. (1, 14)
Limitations of Use
ANDEXXA has not been shown to be effective for, and is not indicated for, the treatment of bleeding related to any FXa inhibitors other than apixaban or rivaroxaban. (1)
Andexxa Dosage and Administration
For intravenous (IV) use only.
Dosage Forms and Strengths
ANDEXXA is available as a lyophilized powder in single-use vials of 200 mg of coagulation factor Xa (recombinant), inactivated-zhzo. (3)
Contraindications
None. (4)
Warnings and Precautions
- •
- Arterial and venous thromboembolic events, ischemic events, and cardiac events, including sudden death, have occurred during treatment with ANDEXXA. Resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA. (5.1)
- •
- Re-elevation or incomplete reversal of anticoagulant activity can occur. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 5%) in bleeding subjects receiving ANDEXXA were urinary tract infections and pneumonia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
Full Prescribing Information
WARNING: THROMBOEMBOLIC RISKS, ISCHEMIC RISKS, CARDIAC ARREST, AND SUDDEN DEATHS
Treatment with ANDEXXA has been associated with serious and life-threatening adverse events, including: (5.1)
- •
- Arterial and venous thromboembolic events
- •
- Ischemic events, including myocardial infarction and ischemic stroke
- •
- Cardiac arrest
- •
- Sudden deaths
Monitor for thromboembolic events and initiate anticoagulation when medically appropriate. Monitor for symptoms and signs that precede cardiac arrest and provide treatment as needed.
1. Indications and Usage for Andexxa
ANDEXXA is indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
This indication is approved under accelerated approval based on the change from baseline in anti-FXa activity in healthy volunteers [see Clinical Studies (14)]. An improvement in hemostasis has not been established. Continued approval for this indication may be contingent upon the results of studies that demonstrate an improvement in hemostasis in patients.
2. Andexxa Dosage and Administration
For intravenous (IV) use only.
2.1 Dose
There are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established.
| Dose* | Initial IV Bolus | Follow-On IV Infusion | Total Number of 200 mg Vials |
|---|---|---|---|
|
|||
|
Low Dose |
400 mg at a target rate of 30 mg/min |
4 mg/min for 120 minutes (480 mg) |
5 |
|
High Dose |
800 mg at a target rate of 30 mg/min |
8 mg/min for 120 minutes (960 mg) |
9 |
The recommended dosing of ANDEXXA is based on the specific FXa inhibitor, dose of FXa inhibitor, and time since the patient's last dose of FXa inhibitor (see Table 2).
- Table 2: ANDEXXA Dose Based on Rivaroxaban or Apixaban Dose (Timing of Last Dose of FXa Inhibitor before ANDEXXA Initiation)
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
2.2 Reconstitution
- •
- The reconstituted solution contains coagulation factor Xa (recombinant), inactivated-zhzo at a concentration of 10 mg/mL.
- •
- Reconstituted ANDEXXA in vials is stable at room temperature for up to eight hours, or may be stored for up to 24 hours at 2°C to 8°C (36°F to 46°F)
- •
- Reconstituted ANDEXXA in IV bags is stable at room temperature for up to eight hours.
IV Bolus Preparation
|
Determine total number of vials required (see Table 1). (or smaller in diameter, e.g. 21-gauge) needle. |  |
|
|
To ensure dissolution of the cake or powder, gently swirl each vial until complete dissolution of powder occurs (A). Do not shake (B); shaking could lead to foaming. Typical dissolution time for each vial is approximately three to five minutes. If dissolution is incomplete, discard the vial, and do not use the product. |
(A) |
(B) |
 |  |
|
|
Use 40-mL (or larger) syringe with a 20-gauge (or smaller in diameter, e.g. 21-gauge) needle to withdraw the reconstituted ANDEXXA solution from each of the vials until the required dosing volume is achieved. Note the total volume withdrawn into the syringe. | 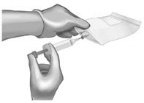 |
|
Discard the syringe and needle.
Discard the vials, including any unused portion.
Continuous IV Infusion Preparation
- •
- Follow the same procedure outlined above for IV bolus preparation. Reconstitute the total number of vials needed based on the dose requirements. More than one 40 to 60-mL syringe, or an equivalent 100-mL syringe, may be used for transfer of reconstituted solution to the IV bag.
- •
- Infusion will require a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
2.3 Administration
- •
- Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration.
- •
- Administer ANDEXXA intravenously, using a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
- •
- Start the bolus at a target rate of approximately 30 mg/min.
- •
- Within two minutes following the bolus dose, administer the continuous IV infusion for 120 minutes.
2.4 Restarting Anticoagulant Therapy
Patients treated with FXa inhibitor therapy have underlying disease states that predispose them to thromboembolic events. Reversing FXa inhibitor therapy exposes patients to the thrombotic risk of their underlying disease. To reduce the risk of thrombosis, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA.
3. Dosage Forms and Strengths
ANDEXXA is available as a white to off-white lyophilized powder in single-use vials of 200 mg of coagulation factor Xa (recombinant), inactivated-zhzo.
5. Warnings and Precautions
5.1 Thromboembolic and Ischemic Risks
The thromboembolic and ischemic risks were assessed in 419 bleeding subjects in the ANNEXA-4 study who received ANDEXXAand were treated with apixaban or rivaroxaban. There were
45/419 (10.7%) subjects who experienced a thrombotic event. The median time to first event was
10 days, and 17 subjects experienced the event within three days of treatment. Of the 419 subjects
who received ANDEXXA, 266 received at least one anticoagulation dose within 30 days after
treatment as a prophylactic measure. Of these 266 subjects, 14 subjects (5.3%) had a thrombotic
event after resumption [see Adverse Reactions (6.1)].
Monitor subjects treated with ANDEXXA for signs and symptoms of arterial and venous thromboembolic events, ischemic events, and cardiac arrest. To reduce thromboembolic risk, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA.
The safety of ANDEXXA has not been evaluated in subjects who experienced thromboembolic events or disseminated intravascular coagulation within two weeks prior to the life-threatening bleeding event requiring treatment with ANDEXXA. Safety of ANDEXXA also has not been evaluated in subjects who received prothrombin complex concentrates, recombinant factor VIIa, or whole blood products within seven days prior to the bleeding event.
5.2 Re-elevation or Incomplete Reversal of Anti-FXa Activity
The time course of anti-FXa activity following ANDEXXA administration was consistent among the healthy volunteer studies and the ANNEXA-4 study in bleeding subjects [see Clinical Studies (14)]. Compared to baseline, there was a rapid and substantial decrease in anti-FXa activity corresponding to the ANDEXXA bolus. This decrease was sustained through the end of the ANDEXXA continuous infusion. The anti-FXa activity returned to the placebo levels approximately two hours after completion of a bolus or continuous infusion. Subsequently, the anti-FXa activity decreased at a rate similar to the clearance of the FXa inhibitors.
A total of 114 subjects from ANNEXA-4 were anticoagulated with apixaban or rivaroxaban and
had elevated baseline levels of anti-FXa (>150 ng/mL for apixaban and >300 ng/mL for
rivaroxaban). After administration of ANDEXXA, these subjects experienced decreased anti-FXa
activity levels, with median reductions of 96% for rivaroxaban and 92% for apixaban.
5.3 Use of Heparin Following Administration of ANDEXXA
ANDEXXA may interfere with the anticoagulant effect of heparin.
Use of ANDEXXA as an antidote for heparin has not been established. Avoid use of ANDEXXA for the reversal of direct FXa inhibitors (apixaban and rivaroxaban) prior to heparinization as ANDEXXA may cause unresponsiveness to heparin. If anticoagulation is needed, use an alternative anticoagulant to heparin.
6. Adverse Reactions/Side Effects
The most common adverse reactions (≥ 5%) in bleeding subjects receiving ANDEXXA were urinary tract infections and pneumonia.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates
observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of
another drug and may not reflect the rates observed in clinical practice.
The ANNEXA-4 study was a multinational, prospective, open-label study using ANDEXXA in
subjects presenting with acute major bleeding and who have recently received an FXa inhibitor.
Four hundred and seventy-seven subjects were enrolled and received ANDEXXA. Of these 477
subjects, 419 subjects were treated with apixaban (245/419; 58.5%) or rivaroxaban (174/419;
41.5%). Most subjects had received apixaban or rivaroxaban as anticoagulation treatment for atrial
fibrillation (348/419; 83%) or venous thromboembolism (65/419; 16%). In the majority of subjects,
ANDEXXA was used to reverse anticoagulant therapy following either an intracranial hemorrhage
(289/419; 69%) or a gastrointestinal bleed (95/419; 23%), with the remaining subjects (35/419;
8.4%) experiencing bleeding at other sites. Subjects were assessed at a Day 30 follow-up visit
following infusion with ANDEXXA.
Deaths
In the ANNEXA-4 study, of the 419 subjects in the safety population who were treated with
apixaban or rivaroxaban, there were 75 deaths (18%). There were 37 cardiovascular deaths related
to bleeding, 19 deaths that were cardiovascular and not related to bleeding, 14 that were non-cardiovascular,
and 5 deaths had an uncertain or unknown cause. The average time to death was 15
days after treatment. All subjects died prior to Day 45. Of the 75 subjects who died, the initial
bleeding event was intracranial bleeding in 55 (73%), gastrointestinal bleeding in 14 (19%), and
other bleeding types in 6 (8%) subjects.
Thromboembolic and Ischemic Events
In the ANNEXA-4 study, 45/419 (10.7%) subjects experienced one or more of the following
thromboembolic events: cerebrovascular accident (CVA) (19/45; 42%), deep venous thrombosis
(11/45; 24%), myocardial infarction (9/45; 20%), pulmonary embolism (5/45; 11%), and transient
ischemic attack (1/45; 2%). The median time to event was ten days. A total of 38% of subjects
with thromboembolic events (17/45) experienced the thromboembolic event during the first three
days. Of the 419 subjects who received ANDEXXA, 282 (67.3%) received any form of re-anticoagulation
within 30 days after treatment. Of these 282 subjects, 16 received anticoagulation
in response to a thrombotic event, while 266 received the anticoagulation as a prophylactic. Of
these 266, 14 subjects (5.3%) had a thrombotic event after resumption of anticoagulation; while of
the 153 subjects who did not receive anticoagulation as a prophylactic, 31 (20.3%) had a
thrombotic event. No patient had a thrombotic event after resumption of oral anticoagulation [see
Warnings and Precautions (5.1)].
Infusion-Related Reactions
In the ANNEXA-4 study, 2/419 (0.5%) subjects experienced an infusion-related reaction, neither
of which were assessed as severe (1 moderate; 1 mild). Reported signs and symptoms were
transient and included rigors, chills, hypertension, oxygen desaturation, agitation and confusion.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of ANDEXXA in pregnant women to inform patients of associated risks. Animal reproductive and developmental studies have not been conducted with ANDEXXA.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ANDEXXA in human milk, the effects on the breastfed child, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ANDEXXA and any potential adverse effects on the breastfed child from ANDEXXA or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of ANDEXXA in the pediatric population have not been studied.
8.5 Geriatric Use
Of the 419 subjects in the ANNEXA-4 study of ANDEXXA, 381 (91%) were 65 years of age or
older, and 278 (66%) were older than 75 years of age. No overall differences in safety or efficacy
were observed between these subjects and younger subjects, and other reported clinical experience
has not identified differences in responses between elderly and younger subjects; however, greater
sensitivity of some older individuals cannot be ruled out.
11. Andexxa Description
ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a sterile, white to off-white lyophilized powder available in single-use vials.
Each 200 mg vial delivers 200 mg of coagulation factor Xa formulated with the inactive ingredients tromethamine (Tris base), Tris hydrochloride, L-arginine hydrochloride, sucrose (1% w/v), mannitol (2.5% w/v), and polysorbate 80 (0.01% w/v) at pH 7.8.
After reconstitution of the lyophilized powder with SWFI for IV administration, the product is a clear, colorless to slightly yellow solution. ANDEXXA contains no preservatives.
The active ingredient in ANDEXXA is a genetically modified variant of human FXa. The active site serine was substituted with alanine, rendering the molecule unable to cleave and activate prothrombin. The gamma-carboxyglutamic acid (Gla) domain was removed to eliminate the protein's ability to assemble into the prothrombinase complex, thus removing the potential anticoagulant effects.
No additives of human or animal origin are used in the manufacture of ANDEXXA. The recombinant protein is produced in a genetically engineered Chinese Hamster Ovary (CHO) cell expression system and has a molecular weight of approximately 41 kDa. The manufacturing process incorporates two validated virus clearance steps.
12. Andexxa - Clinical Pharmacology
12.1 Mechanism of Action
Coagulation factor Xa (recombinant), inactivated-zhzo exerts its procoagulant effect by binding and sequestering the FXa inhibitors, rivaroxaban and apixaban. Another observed procoagulant effect of the ANDEXXA protein is its ability to bind to, and inhibit the activity of, Tissue Factor Pathway Inhibitor (TFPI). Inhibition of TFPI activity can increase tissue factor (TF)-initiated thrombin generation.
12.2 Pharmacodynamics
The effects of ANDEXXA can be measured using assays for its anti-FXa activity, free fraction of FXa inhibitor, and thrombin generation. In addition to its ability to sequester the FXa inhibitors, rivaroxaban and apixaban, ANDEXXA has been shown to inhibit TFPI activity.
The dose and dosing regimen of ANDEXXA that are required to reverse anti-FXa activity and to restore thrombin generation were determined in dose-ranging studies on healthy volunteers. Dosing of ANDEXXA, as a bolus followed by a two-hour continuous infusion, resulted in a rapid decrease in anti-FXa activity (within two minutes after the completion of the bolus administration) followed by reduced anti-FXa activity that was maintained throughout the duration of the continuous infusion [see Clinical Studies (14)]. The anti-FXa activity returned to the placebo levels approximately two hours after completion of a bolus or continuous infusion, whereas TFPI activity in plasma returned to the pretreatment levels approximately 96 hours following ANDEXXA administration.
Elevation of TF-initiated thrombin generation above the baseline range (prior to anticoagulation) occurred within two minutes following a bolus administration of ANDEXXA and was maintained throughout the duration of the continuous infusion. The TF-initiated thrombin generation was elevated above placebo for at least 22 hours. The sustained elevation of thrombin generation over the baseline range and the sustained elevation over placebo were not observed in a contact-activated thrombin generation assay (an assay that is not affected by TF-TFPI interaction).
Laboratory assessment of coagulation does not necessarily correlate with or predict the hemostatic effectiveness of ANDEXXA.
12.2.1 Therapeutic Monitoring
Current commercial clinical anti-FXa-activity assays are unsuitable for measuring FXa activity following administration of ANDEXXA. Due to the reversible binding of ANDEXXA to the FXa inhibitor, the high sample dilution currently used in commercial clinical assays promotes dissociation of the inhibitor from ANDEXXA, resulting in detection of erroneously elevated anti-FXa activity levels, thereby causing a substantial underestimation of the reversal activity of ANDEXXA.
12.3 Pharmacokinetics
A summary of the pharmacokinetic (PK) properties of ANDEXXA in healthy subjects is shown in the table below (see Table 3).
| Low Dose | High Dose | |
|---|---|---|
| Data presented are geometric mean (Geometric Mean % Coefficient of Variation), [range]. | ||
|
n |
11 |
10 |
|
AUC0-∞ (hr*µg/mL) |
200.5 (16.3) |
572.9 (16.0) |
|
Cmax (µg/mL) |
76.6 (17.5) |
206.6 (18.8) |
|
Clearance (L/hr) |
4.4 (16.3) |
3.1 (16.0) |
|
T1/2 (hr) |
3.3 (15.0) |
2.7 (20.0) |
|
Vss (L) |
4.4 (17.6) |
3.0 (23.3) |
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and
specificity of the assay. Differences in assay methods preclude meaningful comparisons of the
incidence of ADAs in the studies described below with the incidence of ADAs in other studies,
including those of ANDEXXA.
Using electrochemiluminescence (ECL)-based assays, 417 ANDEXXA-treated healthy subjects
were tested for antibodies to ANDEXXA as well as for antibodies cross-reacting with factor X
(FX) and FXa. The analysis of available samples up to Day 48 from pooled healthy subjects
revealed low incidence of anti-ANDEXXA (≤ 8.7%) and anti-FXa (≤ 0.8%) at all timepoints and
no anti-FX antibody was detected at any timepoint. The pattern of immunogenicity in subjects with
acute major bleeding in the ANNEXA-4 study was similar to that observed in healthy subjects. Of
the 277 ANDEXXA-treated subjects in ANNEXA-4 who had available samples at Day 30 or 45,
7.9% (22/277) had antibodies against ANDEXXA, 0.4% (1/277) subjects had antibodies against
FXa and no subjects had antibodies against FX. No neutralizing antibodies against ANDEXXA or
cross-reacting with FX or FXa were detected in healthy subjects or in bleeding subjects.
Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the
pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of ANDEXXA have not been
fully characterized.
14. Clinical Studies
The safety and efficacy of ANDEXXA were evaluated in two prospective, randomized, placebo-controlled
studies, conducted in healthy volunteers (Study 1 ANNEXA-A; Study 2 ANNEXA-R).
Both studies examined the percent change in anti-FXa activity, from baseline to nadir, for the low-dose
and high-dose regimens of bolus followed by continuous infusion. Nadir is defined as the
smallest value measured within five minutes after the end of the continuous infusion.
The safety and efficacy of ANDEXXA were evaluated in a multinational, prospective, single-arm,
open-label study (Study 3 ANNEXA-4) in subjects presenting with acute major bleeding and who
have recently received an FXa inhibitor. This study examined the percent change in anti- FXa
activity from baseline to the nadir between five minutes after the end of the bolus up until the end of the infusion and the rate of effective hemostasis within 12 hours after infusion, as rated by an independent endpoint adjudication committee.
Study 1 ANNEXA-A (NCT02207725) – apixaban reversal
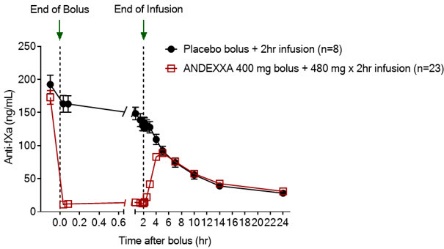
In Study 1, healthy subjects (median age: 57 years; range: 50 to 73 years) received apixaban 5 mg twice daily for three and a half days to achieve steady-state. At three hours after the last apixaban dose (~ Cmax), ANDEXXA or placebo was administered. Eight subjects received placebo, and 24 received ANDEXXA, administered as a 400 mg IV bolus followed by a 4 mg per minute continuous infusion for 120 minutes (total 480 mg).
Study 2 ANNEXA-R (NCT02220725) – rivaroxaban reversal
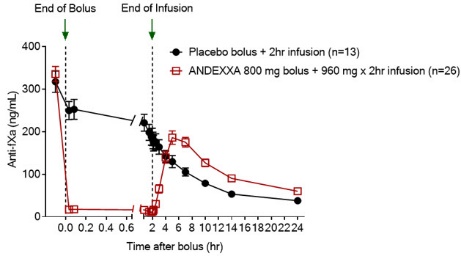
In Study 2, healthy subjects (median age: 57 years; range: 50 to 68 years) received rivaroxaban 20 mg once per day for four days to achieve steady-state. At four hours after the last rivaroxaban dose (~ Cmax), ANDEXXA or placebo was administered. Thirteen subjects received placebo, and 26 received ANDEXXA, administered as an 800 mg IV bolus followed by an 8 mg per minute continuous infusion for 120 minutes (total 960 mg).
Reduction in Anti-FXa Activity
In Study 1 and Study 2, the percent change from baseline in anti-FXa activity at its nadir was statistically significant (p < 0.0001) in favor of the ANDEXXA groups compared to placebo in both Studies 1 and 2. The results of Study 1 and Study 2 are provided below (see Table 4).
The time courses of anti-FXa activity before and after ANDEXXA administration are shown in Figure 1.
| Anti-FXa Activity | ANDEXXA
n=23 | Placebo
n=8 |
|---|---|---|
|
||
|
Mean baseline ng/mL (± SD) |
173.0 |
191.7 |
|
Mean ng/mL (± SD) change from baseline at the nadir* |
-160.6 |
-63.2 |
|
Mean % (± SD) change from baseline at the nadir* |
-92.3 |
-32.7 |
|
Median difference and associated 95% confidence interval (CI)† |
-59.5 (-64.1; -55.2) |
not applicable |
|
p-value |
< 0.0001‡ |
not applicable |
| Anti-FXa Activity | ANDEXXA
n=26 | Placebo
n=13 |
|||
|---|---|---|---|---|---|
| SD = Standard deviation. Note: Baseline is the last assessment obtained prior to the first dose of ANDEXXA or placebo. |
|||||
|
|||||
|
Mean baseline ng/mL (± SD) |
335.3 |
317.2 |
|||
|
Mean ng/mL (± SD) change from baseline at the nadir* |
-324.5 |
-143.4 |
|||
|
Mean % (± SD) change from baseline at the nadir* |
-96.7 |
-44.6 |
|||
|
Median difference and associated 95% confidence interval (CI)† |
-51.9 (-58.0; -47.0) |
not applicable |
|||
|
p-value |
< 0.0001‡ |
not applicable |
|||
Figure 1: Change in Anti-FXa Activity (ng/mL) in Subjects Anticoagulated with Apixaban (A – Study 1) and Rivaroxaban (B – Study 2)
Anti-FXa activity was measured prior to and after ANDEXXA or placebo administration.
Dashed lines indicate the end of the bolus or infusion. A break in the x-axis is added to better visualize the immediate, short-term dynamics of anti-FXa activity following ANDEXXA treatment. The points on the graph represent the mean anti-FXa activity level; error bars illustrate standard error. There was a statistically significant difference (p < 0.05) in the percent change of anti-FXa activity normalized to pre-bolus between ANDEXXA and placebo until two hours after administration of infusion.
A. Apixaban – with ANDEXXA 400 mg IV bolus plus 4 mg/min infusion for 120 minutes.
B. Rivaroxaban – with ANDEXXA 800 mg IV bolus plus 8 mg/min infusion for 120 minutes.
Study 3 ANNEXA-4 (NCT02329327)
In a multinational, prospective, single-arm, open-label study, ANDEXXA was administered to 477
subjects taking FXa inhibitors who presented with acute major bleeding.
The co-primary endpoints are: (a) percent change in anti-FXa activity from baseline to the nadir
between five minutes after the end of the bolus up until the end of the infusion; and (b) rate of
effective hemostasis within 12 hours after infusion, as rated by an independent endpoint
adjudication committee.
For the 477 subjects dosed with ANDEXXA, 302 were efficacy-evaluable defined as subjects (1)
on treatment with apixaban or rivaroxaban; (2) who had a baseline anti-FXa activity above 75
ng/mL; and (3) were adjudicated as meeting eligibility criteria for acute major bleeding [also see
For anti-FXa activity, the median (95% CI) decrease from baseline to nadir in anti-FXa activity for
apixaban was -93% (-94%; -92%) and for rivaroxaban was -94% (-95%; -93%).
An improvement in hemostasis has not been established. ANDEXXA has not been shown to be
effective for bleeding related to any FXa inhibitors other than apixaban or rivaroxaban.
16. How is Andexxa supplied
How Supplied
ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a white to off-white lyophilized cake or powder supplied as single-use vials in a carton. ANDEXXA is not made with natural rubber latex.
ANDEXXA vials are provided as follows (see Table 5):
| NDC | Carton Configuration | Vial Cap Color | Packaging Color |
|---|---|---|---|
|
NDC 0310-3200-04 |
4 single use vials in a carton, each vial containing 200 mg of ANDEXXA |
Vials have a red flip-off cap. |
|
|
NDC 0310-3200-05 |
5 single use vials in a carton, each vial containing 200 mg of ANDEXXA |
17. Patient Counseling Information
Inform patients that reversing FXa inhibitor therapy increases the risk of thromboembolic events. Arterial and venous thromboembolic events, ischemic events, cardiac events, and sudden death were observed within 30 days following ANDEXXA administration [see Warnings and Precautions (5.1)].
| ANDEXXA
andexanet alfa injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |
Frequently asked questions
More about Andexxa (coagulation factor Xa)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: anticoagulant reversal agents
- En español