Andexxa Dosage
Generic name: andexanet alfa 200mg in 20mL
Dosage form: injection, powder, lyophilized, for solution
Drug class: Anticoagulant reversal agents
Medically reviewed by Drugs.com. Last updated on May 23, 2025.
Dose
For intravenous (IV) use only.
There are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established.
| Dose* | Initial IV Bolus | Follow-On IV Infusion | Total Number of 200 mg Vials |
|---|---|---|---|
|
|||
|
Low Dose |
400 mg at a target rate of 30 mg/min |
4 mg/min for 120 minutes (480 mg) |
5 |
|
High Dose |
800 mg at a target rate of 30 mg/min |
8 mg/min for 120 minutes (960 mg) |
9 |
The recommended dosing of ANDEXXA is based on the specific FXa inhibitor, dose of FXa inhibitor, and time since the patient's last dose of FXa inhibitor (see Table 2).
Table 2: ANDEXXA Dose Based on Rivaroxaban or Apixaban Dose (Timing of Last Dose of FXa Inhibitor before ANDEXXA Initiation)
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Reconstitution
- •
- The reconstituted solution contains coagulation factor Xa (recombinant), inactivated-zhzo at a concentration of 10 mg/mL.
- •
- Reconstituted ANDEXXA in vials is stable at room temperature for up to eight hours, or may be stored for up to 24 hours at 2°C to 8°C (36°F to 46°F).
- •
- Reconstituted ANDEXXA in IV bags is stable at room temperature for up to eight hours.
IV Bolus Preparation
|
Determine total number of vials required (see Table 1). (or smaller in diameter, e.g., 21-gauge) needle. |
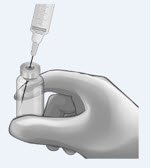 |
|
|
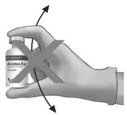
To ensure dissolution of the cake or powder, gently swirl each vial until complete dissolution of powder occurs (A). Do not shake (B); shaking could lead to foaming. Typical dissolution time for each vial is approximately three to five minutes. If dissolution is incomplete, discard the vial, and do not use the product. |
(A) |
(B) |
 |
 |
|
|
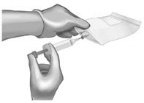
Use 40-mL (or larger) syringe with a 20-gauge (or smaller in diameter, e.g., 21-gauge) needle to withdraw the reconstituted ANDEXXA solution from each of the vials until the required dosing volume is achieved. Note the total volume withdrawn into the syringe. |
 |
|
Discard the syringe and needle.
Discard the vials, including any unused portion.
Continuous IV Infusion Preparation
- •
- Follow the same procedure outlined above for IV bolus preparation. Reconstitute the total number of vials needed based on the dose requirements. More than one 40 to 60 mL syringe, or an equivalent 100-mL syringe, may be used for transfer of reconstituted solution to the IV bag.
- •
- Infusion will require a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
Administration
- •
- Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration.
- •
- Administer ANDEXXA intravenously, using a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
- •
- Start the bolus at a target rate of approximately 30 mg/min.
- •
- Within two minutes following the bolus dose, administer the continuous IV infusion for 120 minutes.
Restarting Anticoagulant Therapy
Patients treated with FXa inhibitor therapy have underlying disease states that predispose them to thromboembolic events. Reversing FXa inhibitor therapy exposes patients to the thrombotic risk of their underlying disease. To reduce the risk of thrombosis, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA.
Frequently asked questions
More about Andexxa (coagulation factor Xa)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: anticoagulant reversal agents
- En español
Patient resources
Professional resources
- Andexxa prescribing information
- Coagulation Factor Xa (recombinant), Inactivated-zhzo (AHFS Monograph)
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

