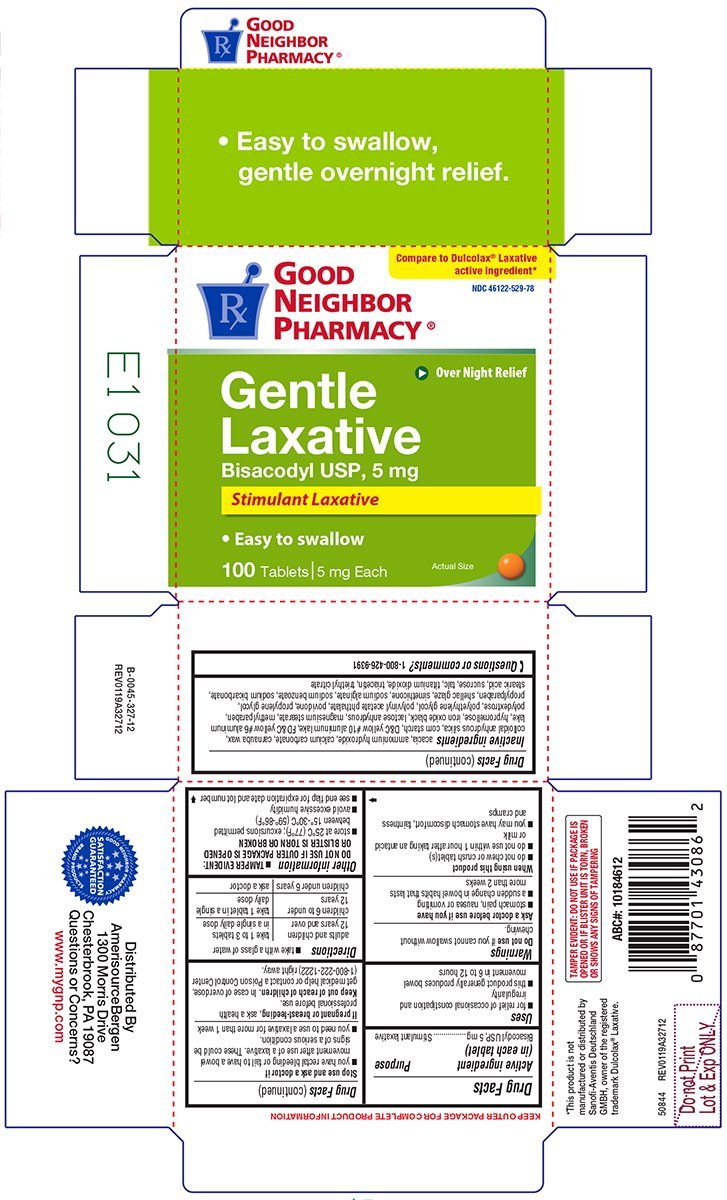
Gentle Laxative
Dosage form: tablet, sugar coated
Ingredients: BISACODYL 5mg
Labeler: Amerisource Bergen
NDC code: 46122-529
Medically reviewed by Drugs.com. Last updated on Mar 11, 2024.
Bisacodyl USP, 5 mg
Stimulant laxative
- for relief of occasional constipation and irregularity
- this product generally produces bowel movement in 6 to 12 hours
if you cannot swallow without chewing.
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- you may have stomach discomfort, faintness and cramps
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- take with a glass of water
| adults and children 12 years and over | take 1 to 3 tablets in a single daily dose |
| children 6 to under 12 years | take 1 tablet in a single daily dose |
| children under 6 years | ask a doctor |
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
- avoid excessive humidity
- see end flap for expiration date and lot number
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
1-800-426-9391
GOOD
NEIGHBOR
PHARMACY®
Compare to Dulcolax® Laxative
active ingredient*
NDC 46122-529-78
Over Night Relief
Gentle
Laxative
Bisacodyl USP, 5 mg
Stimulant Laxative
• Easy to swallow
100 Tablets | 5 mg Each
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or
distributed by Sanofi-Aventis
Deutschland GMBH, owner of the
registered trademark Dulcolax® Laxative.
50844 REV0119A32712
Distributed by
AmerisourceBergen
1300 Morris Drive
Chesterbrook, PA 19087
Questions or Concerns?
www.mygnp.com
Good Neighbor Pharmacy Brand Products
SATISFACTION GUARANTEED
| GENTLE LAXATIVE
bisacodyl tablet, sugar coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Amerisource Bergen (007914906) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(46122-529) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(46122-529) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | PACK(46122-529) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(46122-529) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(46122-529) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.