SSANGGI TANG
Dosage form: liquid
Ingredients: PAEONIA LACTIFLORA ROOT 0.9785g in 100mL, REHMANNIA GLUTINOSA ROOT 0.6745g in 100mL, LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT 0.627g in 100mL, ASTRAGALUS GUMMIFER ROOT 0.4845g in 100mL
Labeler: LYDIA Co., Ltd
NDC code: 72988-0022
Medically reviewed by Drugs.com. Last updated on Oct 9, 2023.
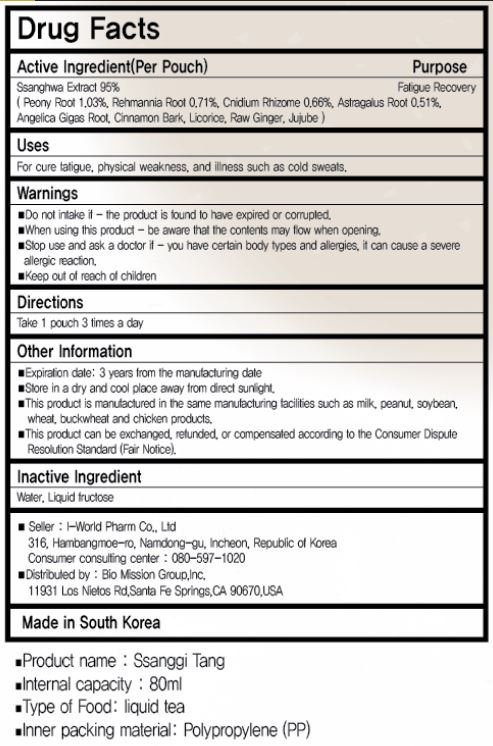
ssanghwa extract (peony root, rehmannia root, cnidium rhizome, astragalus root, angelica gias root, cinnamon bark, licorice, raw ginger, jujube)
for cure fatigue, physical weakness, and illness such as cold sweats
Keep out of reach of children
take 1 pouch 3 times a day
do not intake if the product is found to have expired or corrupted
when using this product be aware that the contents may flow when opening
stop use and ask a doctor if you have certain body types and allergies, it can cause a severe allergic reaction
water, liquid fructose
For oral use only
| SSANGGI TANG
ssanghwa extract liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - LYDIA Co., Ltd (695735569) |
| Registrant - LYDIA Co., Ltd (695735569) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| I World Pharmaceutical Co., Ltd | 688222857 | manufacture(72988-0022) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LYDIA Co., Ltd | 695735569 | label(72988-0022) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.