Biotic-Plus
Dosage form: ointment
Ingredients: bacitracin zinc 400[iU] in 1g, neomycin sulfate 3.5mg in 1g, polymyxin b sulfate 5000[iU] in 1g, lidocaine .40mg in 1g
Labeler: Provision Medical
NDC code: 69103-2400
Medically reviewed by Drugs.com. Last updated on Jul 15, 2024.
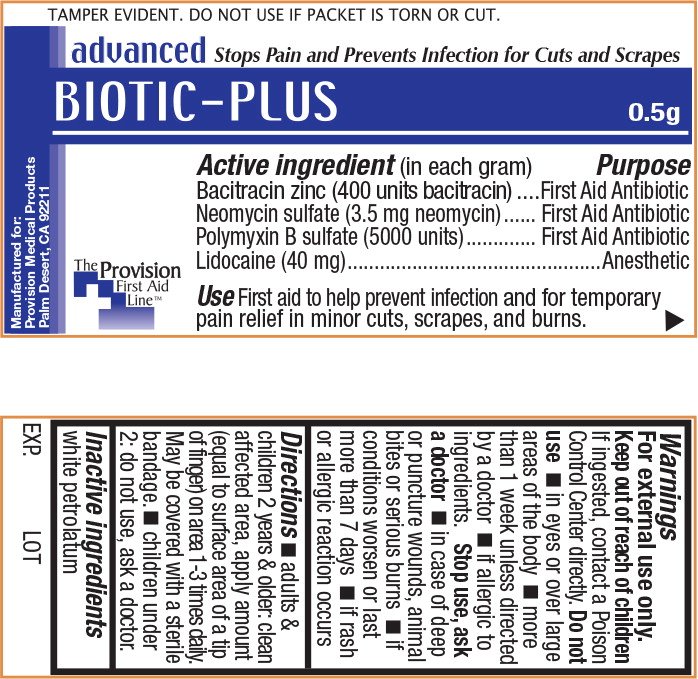
TAMPER EVIDENT. DO NOT USE IF PACKET IS TORN OR CUT.
advanced Stops Pain and Prevents Infection for Cuts and Scrapes
BIOTIC-PLUS 0.5g
(in each gram)
Bacitracin zinc (400 units Bacitracin)
Neomycin sulfate (3.5 mg neomycin)
Polymyxin B sulfate (5000 units)
Lidocaine .40 mg
First Aid Antibiotic
First Aid Antibiotic
First Aid Antibiotic
Anesthetic
First aid to help prevent infection and provide temporary relief in minor cuts, scrapes, and burns
The Provision First Aid Line
Manufactured for Provision Medical Products Palm Desert, CA 92211
For external use only.
If ingested, contact a Poison Control Center directly.
- in eyes or over large areas of the body
- more than 1 week unless directed by a doctor
- if allergic to ingredients.
- in case of deep or puncture wounds, animal bites or serious burns
- if conditions worsen or last more than 7 days
- if rash or allergic reaction occurs
- adults & children 2 years & older: clean affected area, apply amount (equal to surface area of a tip of finger) on area 1-3 times daily. May be covered with a sterile bandage.
- children under 2: do not use, ask a doctor
white petrolatum
| BIOTIC-PLUS
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, lidocaine ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Provision Medical (036936831) |
| Registrant - Safetec of America, Inc. (874965262) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Safetec of America, Inc. | 874965262 | MANUFACTURE(69103-2400) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.