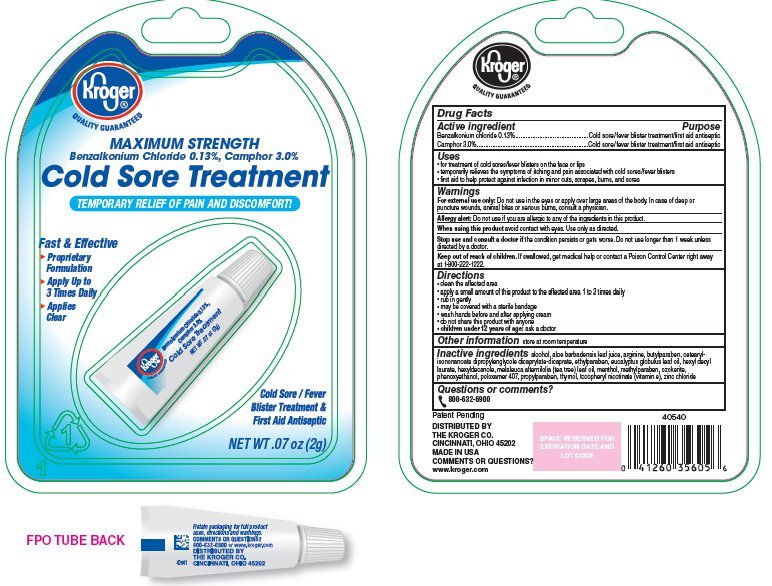
Kroger Cold Sore Treatment
Dosage form: cream
Ingredients: Benzalkonium Chloride 1.3mg in 1g, CAMPHOR (SYNTHETIC) 30mg in 1g
Labeler: The Kroger Co.
NDC code: 30142-408
Medically reviewed by Drugs.com. Last updated on Apr 22, 2024.
Benzalkonium chloride 0.13%
Camphor 3%
Cold sore/fever blister treatment/first aid antiseptic
- •
- for treatment of cold sores/fever blisters on the face or lips
- •
- temporarily relieves the symptoms of itching and pain associated with cold sores/fever blisters
- •
- first aid to help protect against infection in minor cuts, scrapes, burns, and sores
- For external use only: Do not use in the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a physician.
- Allergy Alert: Do not use if you are allergic to any of the ingredients in this product.
- When using this product avoid contact with eyes. Use only as directed.
- Stop use and consult a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
- •
- clean the affected area
- •
- apply a small amount of this product to the affected area 1 to 3 times daily
- •
- rub in gently
- •
- may be covered with a sterile bandage
- •
- wash hands before and after applying cream
- •
- do not share this product with anyone
- •
- children under 12 years of age: ask a doctor
store at room temperature
alcohol, aloe barbadensis leaf juice, arginine, butylparaben, cetearyl isononanoate, dipropylene glycol dicaprylate/dicaprate, ethylparaben, eucalyptus globulus leaf oil, hexyl decyl laurate, hexyldecanole, melaleuca alternifolia (tea tree) leaf oil, menthol, methylparaben, ozokerite, phenoxyethanol, poloxamer 407, propylparaben, thymol, tocopheryl nicotinate (vitamin e), zinc chloride
800-632-6900.
| KROGER COLD SORE TREATMENT
camphor cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - The Kroger Co. (006999528) |
| Registrant - Ranir LLC (364567615) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ranir LLC | 364567615 | RELABEL(30142-408), REPACK(30142-408) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.