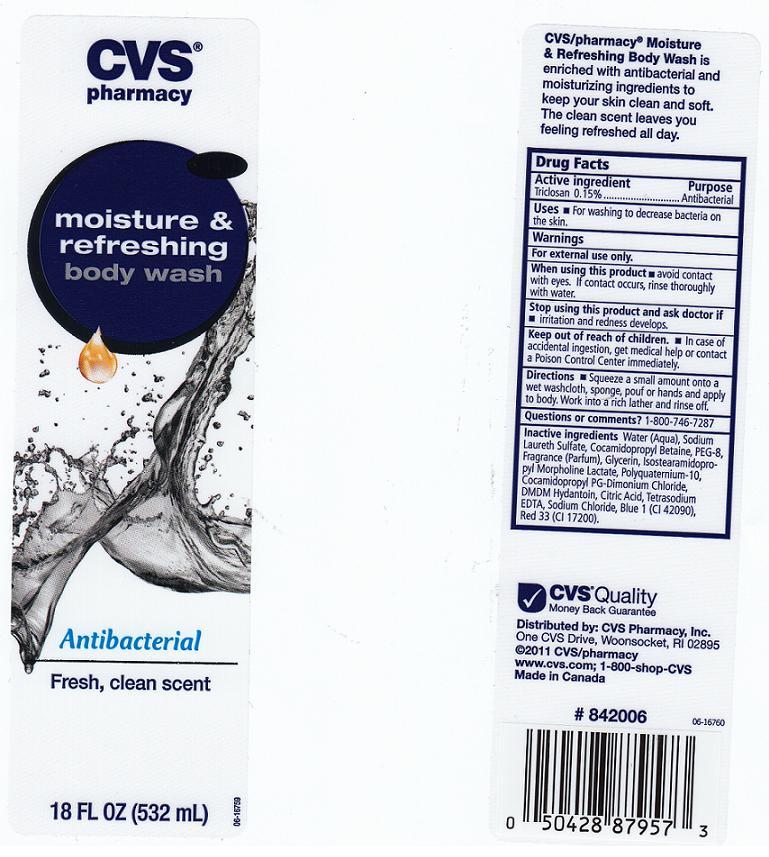
Antibacterial Body Wash
Dosage form: liquid
Ingredients: TRICLOSAN 0.15mL in 100mL
Labeler: CVS Pharmacy
NDC code: 59779-805
Medically reviewed by Drugs.com. Last updated on Jun 17, 2024.
Triclosan 0.15%
For washing to decrease bacteria on the skin.
AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE THROUGHLY WITH WATER.
IRRITATION AND REDNESS DEVELOPS.
IN CASE OF ACCIDENTAL INGESTION, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
SQUEEZE A SMALL AMOUNT ONTO A WET WASHCLOTH, SPONGE, POUF OR HANDS AND APPLY TO BODY. WORK INTO A RICH LATHER AND RINSE OFF.
1-800-746-7287
WATER, SODIUM LAURETH SULFATE, COCAMIDOPROPYL BETAINE, PEG-8, FRAGRANCE, GLYCERIN, ISOSTEARAMIDOPROPYL MORPHOLINE LACTATE, POLYQUATERNIUM-10, COCAMIDOPROPYL PG-DIMONIUM CHLORIDE, DMDM HYDANTOIN, CITRIC ACID, TETRASODIUM EDTA, SODIUM CHLORIDE, BLUE 1 (CI42090), RED 33 (CI 17200).
| ANTIBACTERIAL BODY WASH
triclosan liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Apollo Health and Beauty Care (201901209) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Apollo Health and Beauty Care | 201901209 | manufacture | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.