Fortress
Dosage form: solution
Ingredients: CHLOROXYLENOL 5mg in 1mL
Labeler: Kay Chemical Co.
NDC code: 63146-105
Medically reviewed by Drugs.com. Last updated on Sep 27, 2023.
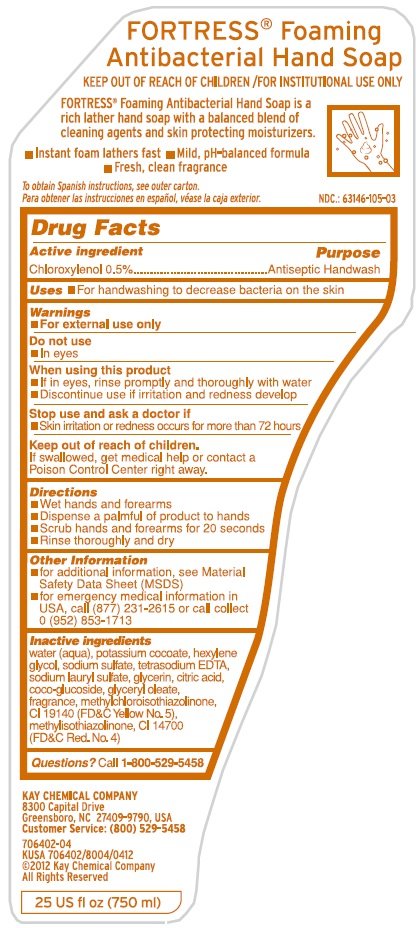
Chloroxylenol 0.5%
Antiseptic Handwash
- For handwashing to decrease bacteria on the skin.
-
For external use only
- In eyes
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
- Skin irritation or redness occurs for more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
- Wet hands and forearms
- Dispense a palmful of product to hands
- Scrub hands and forearms for 20 seconds
- Rinse thoroughly and dry
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA, call (877) 231-2615 or call collect 0 (952) 853-1713
Inactive ingredients: water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium EDTA, sodium lauryl sulfate, glycerin, citric acid, coco-glucoside, glyceryl oleate, fragrance, methychloroisothiazolinone, CI 19140 (FD&C Yellow No. 5), methylisothiazolinone, CI 14700 (FDC Red No. 4)
Questions? Call 1-800-529-5458
FORTRESS® Foaming Antibacterial Hand Soap
KEEP OUT OF REACH OF CHILDREN/FOR INSTITUTIONAL USE ONLY
FORTRESS® Foaming Antibacterial Hand Soap is a rich lather hand soap with a balanced blend of cleaning agents and skin protecting moisturizers.
- Instant foam lathers fast • Mild, pH-balanced formula
- Fresh, clean fragrance
To obtain Spanish instructions, see outer carton.
Para obtener las instrucciones en español, véase la caja exterior.
NDC.: 63146-105-03
KAY CHEMICAL COMPANY
8300 Capital Drive
Greensboro, NC 27409-3790, USA
Customer Service: (800) 529-5458
706402-04
KUSA 706402/8004/0412
©2012 Kay Chemical Company
All Rights Reserved
25 US fl OZ (750 ml)
| FORTRESS
chloroxylenol solution |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Kay Chemical Co. (003237021) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.