OCUVITE Lutein Pill - yellow capsule/oblong
Generic Name: multivitamin with minerals
Pill with imprint OCUVITE Lutein is Yellow, Capsule/Oblong and has been identified as Ocuvite lutein . It is supplied by Bausch & Lomb Inc.
Ocuvite Lutein is used in the treatment of Vitamin/Mineral Supplementation and Deficiency and belongs to the drug class vitamin and mineral combinations. FDA has not classified the drug for risk during pregnancy. Ocuvite Lutein is not a controlled substance under the Controlled Substances Act (CSA).
Images for OCUVITE Lutein
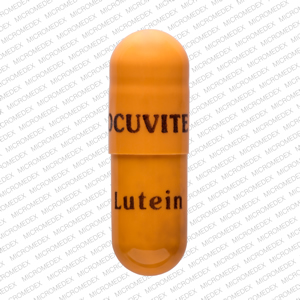
Ocuvite lutein
- Generic Name
- multivitamin with minerals
- Imprint
- OCUVITE Lutein
- Strength
- Color
- Yellow
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Vitamin and mineral combinations
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Bausch & Lomb Inc.
- National Drug Code (NDC)
- 24208-0403
More about Ocuvite Lutein (multivitamin with minerals)
- Check interactions
- Compare alternatives
- Reviews (2)
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
Patient resources
Other brands
Prosteon, Multivitamins and Minerals, Vitafol, Advanced AM/PM, ... +6 more
Professional resources
Other brands
Dolomite, Nicomide, MagneBind 400 Rx, Strovite One, ... +8 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
