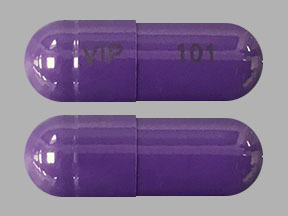
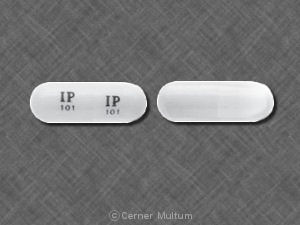
IP 101 IP 101 Pill - white capsule/oblong, 16mm
Pill with imprint IP 101 IP 101 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by Amneal Pharmaceuticals.
Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 100 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for IP 101 IP 101



Gabapentin
- Imprint
- IP 101 IP 101
- Strength
- 100 mg
- Color
- White
- Size
- 16.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Gamma-aminobutyric acid analogs
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Amneal Pharmaceuticals
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 53746-0101 (Discontinued) | Amneal Pharmaceuticals LLC |
| 65162-0101 | Amneal Pharmaceuticals LLC |
| 42291-0300 (Discontinued) | AvKare, Inc. |
| 63739-0675 (Discontinued) | McKesson Packaging Services |
| 63739-0591 | McKesson Packaging Services |
Related images for "IP 101 IP 101"
More about gabapentin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2,514)
- Drug images
- Latest FDA alerts (4)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: gamma-aminobutyric acid analogs
- Breastfeeding
- En español
Patient resources
- Gabapentin drug information
- Gabapentin Capsules
- Gabapentin Oral Solution
- Gabapentin Tablets (PHN)
- Gabapentin Tablets 600 mg and 800 mg
Other brands
Professional resources
- Gabapentin monograph
- Gabapentin (FDA)
- Gabapentin Capsules (FDA)
- Gabapentin Oral Solution (FDA)
- Gabapentin Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.