Thrombate III Dosage
Generic name: Antithrombin Iii Human 50[iU] in 1mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Dec 13, 2023.
For intravenous use after reconstitution only
Dose
- Each vial of THROMBATE III has the functional activity, in International Units (units), stated on the label of the vial. The potency assignment has been determined with a standard calibrated against a World Health Organization antithrombin reference preparation. When prepared as directed, the approximate final concentration is 50 units per milliliter.
- A guide for dosing THROMBATE III is provided in Table 1.
| Regimen (timing) |
Target AT Level |
Dose (Units) | Monitor AT Level |
| Loading Dose* | 120% of normal† | 120 % - baseline % x body weight (kg) 1.4% |
|
| Dose Adjustment* (adjust as needed) |
80% to 120% of normal† | Target % - trough % x body weight (kg) 1.4% |
|
| Maintenance Dose (approximately every 24 hours, adjust as needed) |
80% to 120% of normal† | Loading Dose x 0.6 |
|
- Monitor functional plasma levels of AT. [see table above and Warnings and Precautions (5.3)], and adjust subsequent dosing based on the trough level achieved with the preceding dose until predictable peak and trough levels have been achieved, generally between 80% to 120% of normal.(1)
- Maintain plasma AT levels between 80% to 120% by administering maintenance doses of 60% of the loading dose, administered every 24 hours. Adjust the maintenance dose and interval between doses based on actual plasma AT levels achieved.
- Individualize the exact loading and maintenance dose and/or dose intervals for each patient based on the individual clinical conditions, response to therapy, and actual plasma AT levels achieved. Recovery of THROMBATE III may vary by patient. For example,
- The half-life of AT has been reported to be shortened following surgery,(2) hemorrhage or acute thrombosis, and during intravenous heparin (or low molecular weight heparin) administration.(3-6) In such conditions, monitor plasma AT levels more frequently, and administer THROMBATE III as necessary. [see Warnings and Precautions (5.3), Drug Interactions (7)]
- When an infusion of THROMBATE III is indicated for a patient with hereditary deficiency to control an acute thrombotic episode or prevent thrombosis during or following surgical or obstetrical procedures, raise the AT level to normal and maintain this level for 2 to 8 days, depending on the indication for treatment, type and extent of surgery, patient’s medical condition, past history and physician’s judgment. Base the concomitant administration of heparin in each of these situations on the medical judgment of the physician. [see Drug Interactions (7)]
Reconstitution
- Warm THROMBATE III and Sterile Water for Injection, USP (diluent) vials to room temperature before reconstitution.
- Remove shrink band from the THROMBATE III vial. If the shrink band is absent or shows signs of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
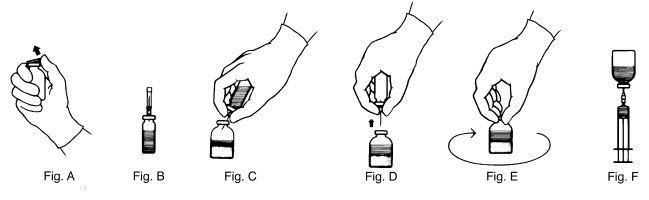
- Remove the plastic flip top from each vial (Fig. A). Cleanse each vial stopper with an alcohol swab and allow surface to dry.
- Carefully remove the plastic sheath from the short end of the transfer needle. Insert the exposed needle into the diluent vial to the hub (Fig. B).
- Carefully grip the sheath of the other end of the transfer needle and twist to remove it.
- Invert the diluent vial and insert the attached needle into the THROMBATE III vial at a 45° angle (Fig. C). This will direct the stream of diluent against the wall of the vial and minimize foaming. The vacuum will draw the diluent into the THROMBATE III vial.*
- When diluent transfer is complete, remove the diluent vial and transfer needle (Fig. D).
- Immediately after adding the diluent, swirl the THROMBATE III vial continuously until the product is completely dissolved (Fig. E). Some foaming may occur, but attempt to avoid excessive foaming. Visually inspect the vial for particulate matter and discoloration prior to administration.
- Clean the top of the vial of reconstituted THROMBATE III again with a new alcohol swab and let surface dry.
- Attach the filter needle (from the package) to the sterile syringe. Withdraw the THROMBATE III solution into the syringe through the filter needle (Fig. F).
- Remove the filter needle from the syringe and replace with an appropriate injection or butterfly needle for administration.
- If the same patient is using more than one vial of THROMBATE III, draw the contents of multiple vials into the same syringe through the filter needles provided.
- *
- If vacuum is lost in the THROMBATE III vial during reconstitution, use a sterile syringe to remove the sterile water from the diluent vial and inject it into the THROMBATE III vial, directing the stream of fluid against the wall of the vial.
Administration
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Administer THROMBATE III, once reconstituted, alone without mixing with other agents or diluents.
- Administer within 3 hours following reconstitution. Do not refrigerate after reconstitution.
- Adapt the rate of administration to the response of the individual patient, but administration of the entire dose in 10 to 20 minutes is generally well tolerated.
More about Thrombate III (antithrombin iii)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- Drug class: miscellaneous coagulation modifiers
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.