RUBY-FILL Dosage
Generic name: RUBIDIUM CHLORIDE RB-82 100mCi
Dosage form: injection, solution
Drug class: Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Oct 10, 2023.
Radiation Safety - Drug Handling
Rubidium Rb 82 is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.3]).
- Use only additive-free 0.9% Sodium Chloride Injection USP to elute the generator [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1)].
- Use waterproof gloves and effective shielding when handling rubidium Rb 82 chloride injection and the RUBY Rubidium Elution System.
- Use aseptic techniques in all drug handling.
- Visually inspect the drug for particulate matter and discoloration prior to administration whenever solution and container permit. Do not administer eluate from the generator if there is any evidence of foreign matter.
Recommended Dose and Administration Instructions
- The recommended weight-based dose of rubidium Rb 82 chloride to be administered per rest or stress component of a PET myocardial perfusion imaging (MPI) procedure is between 10 to 30 Megabecquerels (MBq)/kg [0.27 to 0.81 millicuries (mCi)/kg].
- Do not exceed a single dose of 2220 MBq (60 mCi).
- Use the lowest dose necessary to obtain adequate cardiac visualization and individualize the weight-based dose depending on multiple factors, including, patient weight, imaging equipment and acquisition type used to perform the procedure. For example, 3D imaging acquisition may require doses at the lower end of the recommended range compared to 2D imaging.
- Administer the single dose at a rate of 15 to 30 mL/minute through a catheter inserted into a large peripheral vein; do not exceed an infusion volume of 60 mL.
- Instruct patients to void as soon as a study is completed and as often as possible thereafter for at least one hour.
- The maximum available activity (delivery limit) will decrease as the generator ages [see Dosage and Administration (2.8)].
Image Acquisition Guidelines
For Rest Imaging:
- Administer a single (“rest”) rubidium Rb 82 chloride dose;
- Start imaging 60 to 90 seconds after completion of the infusion of the rest dose and acquire images for 3 to 7 minutes.
For Stress Imaging:
- Begin the study 10 minutes after completion of the resting dose infusion, to allow for sufficient Rb 82 decay;
- Administer a pharmacologic stress agent in accordance with its prescribing information;
- After administration of the pharmacologic stress agent, administer the second dose of Rb 82 at the time interval according to the prescribing information of the pharmacological stress agent;
- Start imaging 60 to 90 seconds after completion of the stress rubidium Rb 82 chloride dose infusion and acquire images for 3 to 7 minutes.
For Both Rest and Stress Imaging:
- If a longer circulation time is anticipated (e.g., in a patient with severe left ventricular dysfunction), start imaging 120 seconds after the rest dose.
- Acquisition may be started immediately post-injection if dynamic imaging is needed.
Elution System
- Use RUBY-FILL Rubidium Rb 82 Generator only with an elution system specifically designed for use with the generator (RUBY Rubidium Elution System) and capable of accurate measurement and delivery of doses of rubidium Rb 82 chloride injection.
- The generator used with the elution system provides ± 10% accuracy for rubidium Rb 82 chloride doses between 370 to 2220 MBq (10 to 60 mCi)
- Follow instructions in the RUBY Rubidium Elution System User Manual for the set up and intravenous infusion of rubidium Rb 82 chloride injection dose.
Directions for Eluting Rubidium Rb 82 Chloride Injection
- Use only additive-free 0.9 % Sodium Chloride Injection USP to elute the generator [see Boxed Warning, Contraindications (4) and Warnings and Precautions (5.1)].
- Prepare the 0.9% Sodium Chloride Injection USP for use with the Saline Confirmation Label
- Affix the saline confirmation label provided with the RUBY Rubidium Elution System on the clear side of the additive-free 0.9% Sodium Chloride Injection USP bag and install on the RUBY Rubidium Elution System.
- Prepare the intravenous administration port in accordance with the DOSAGE AND ADMINISTRATION section of the approved prescribing information of the 0.9% Sodium Chloride Injection USP.
- The port of the sodium chloride bag must be penetrated only one time.
- Once the bag port is penetrated, it should remain installed on the RUBY Rubidium Elution System for its entire period of use. A maximum use time of 12 hours from the initial port penetration is permitted.
- Before the next patient, replace the saline bag as part of the mandatory daily quality control procedure.
- Allow at least 10 minutes between elutions for regeneration of Rb 82.
- The system will automatically discard the first 75 mL eluate each day the generator is first eluted.
- The RUBY Rubidium Elution System automatically generates records and saves data of all eluate volumes (from flushing, QC testing, patient infusions), representing the cumulative volume of eluate from the generator.
2.6 Quality Control Testing Procedure
- Elute with additive-free 0.9% Sodium Chloride Injection USP only. [see Boxed Warning, Contraindications (4) and Warnings and Precautions (5.1)].
- Replace the saline bag daily as part of the mandatory daily quality control procedure.
- Use the ionization chamber-type dose calibrator with the elution system (used specifically with the RUBY-FILL Rubidium Rb 82 Generator) for eluate testing.
- Perform Mandatory Eluate Testing (i.e. Quality Control Testing Procedure) to determine Rb 82, Sr 82, and Sr 85 levels:
- Daily - Before administering rubidium Rb 82 chloride injection to the first patient each day.
- Repeat Every 4 patients after an Alert Limit has been detected.
Alert Limits:- 20 L total elution volume has passed through the generator column, or
- Sr 82 level reaches 0.004 mcCi per mCi (kBq per MBq) Rb 82, or
- Sr 85 level reaches 0.04 mcCi per mCi (kBq per MBq) Rb 82.
- Immediately after detection of the volume alert limit (20 L).
- The elution system will automatically indicate when alert limits have been reached and require that additional tests be performed.
When the Quality Control Testing Procedure is performed as described in the User Manual, the system automatically performs the following eluate testing:
Rubidium Eluate Testing:
- The dose calibrator is automatically set for Rb 82 within the Elution System.
- The Quality Control test begins by automatically initiating a generator flush using 75 mL of 0.9% Sodium Chloride Injection USP. This eluate is by default diverted towards the waste container and is ultimately discarded.
- After the generator flush, the system waits approximately 15.2 minutes to accomplish a complete generator recharge of 12 Rb 82 half-lives
- The system then elutes a calibration sample (35 mL of 0.9% Sodium Chloride Injection USP at 20 mL/min). Using the dose calibrator, the system automatically quantifies the activity of Rb 82 in the calibration sample (Rb 82 decay does not need to be corrected for because of a real-time automated measurement).
Strontium Eluate Testing (Strontium Breakthrough):
- Using the calibration sample obtained from the Rb 82 eluate testing, the system allows the sample to stand for 30 minutes to allow for the complete decay of Rb 82.
- The system measures the activity of the sample to automatically determine the total Sr 82 and Sr 85 activity.
- The system automatically determines the ratio (R) on the day (post calibration) of the measurement using the ratio of Sr 85/Sr 82 on the day of calibration provided on the generator label and the Sr 85/Sr 82 ratio factor from the Sr 85/Sr 82 ratio based on generator age using the following equation:

4. The system uses a correction factor (F) of 0.48 to compensate for the contribution of Sr 85 to the reading.
5. The system calculates the amount of Sr 82 in the sample using the following equation:
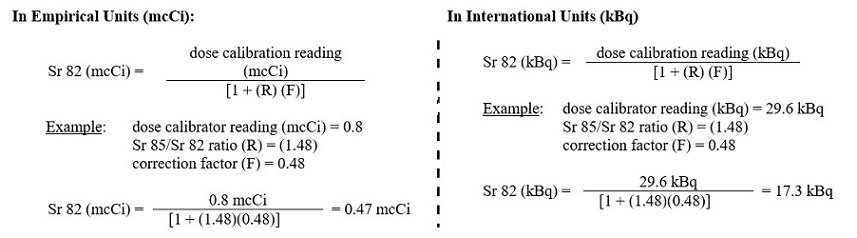
6. The system determines if Sr 82 in the eluate exceeds an Alert or Expiration Limit by dividing the mcCi (or kBq) of Sr 82 by the mCi (or MBq) of Rb 82 at End of Elution (see below for further instructions based on the Sr 82 level)
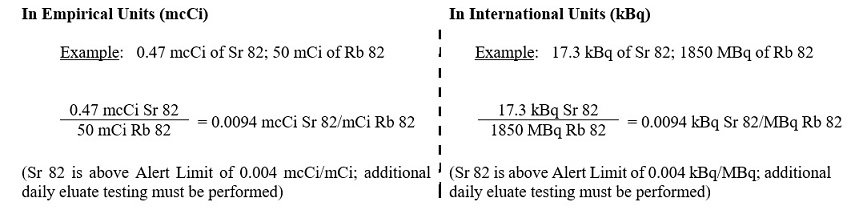
7. The system determines if Sr 85 in the eluate exceeds an Alert or Expiration Limit by multiplying the result obtained in step 6 by (R) as calculated in step 3 (above).

The system uses Table 1 to calculate the decay factor for Rb 82
TABLE 1 |
|||
Physical Decay Chart: Rb 82 half-life 75 seconds |
|||
Seconds |
Fraction Remaining |
Seconds |
Fraction Remaining |
0* |
1.00 |
165 |
0.218 |
15 |
0.871 |
180 |
0.190 |
30 |
0.758 |
195 |
0.165 |
45 |
0.660 |
210 |
0.144 |
60 |
0.574 |
225 |
0.125 |
75 |
0.500 |
240 |
0.109 |
90 |
0.435 |
255 |
0.095 |
105 |
0.379 |
270 |
0.083 |
120 |
0.330 |
285 |
0.072 |
135 |
0.287 |
300 |
0.063 |
150 |
0.250 |
||
*Elution time
The system uses Table 2 to calculate the ratio (R) of Sr 85/Sr 82.
|
Sr 85/Sr 82 Ratio Chart (Sr 85 T1/2 = 65 days, Sr 82 T1/2 = 25 days) |
|||||
| Days | RatioFactor | Days | Ratio Factor | Days | Ratio Factor |
0* |
1.00 |
21 |
1.43 |
42 |
2.05 |
1 |
1.02 |
22 |
1.46 |
43 |
2.08 |
2 |
1.03 |
23 |
1.48 |
44 |
2.12 |
3 |
1.05 |
24 |
1.51 |
45 |
2.15 |
4 |
1.07 |
25 |
1.53 |
46 |
2.19 |
5 |
1.09 |
26 |
1.56 |
47 |
2.23 |
6 |
1.11 |
27 |
1.58 |
48 |
2.27 |
7 |
1.13 |
28 |
1.61 |
49 |
2.30 |
8 |
1.15 |
29 |
1.64 |
50 |
2.34 |
9 |
1.17 |
30 |
1.67 |
51 |
2.38 |
10 |
1.19 |
31 |
1.70 |
52 |
2.43 |
11 |
1.21 |
32 |
1.73 |
53 |
2.47 |
12 |
1.23 |
33 |
1.76 |
54 |
2.51 |
13 |
1.25 |
34 |
1.79 |
55 |
2.55 |
14 |
1.27 |
35 |
1.82 |
56 |
2.60 |
15 |
1.29 |
36 |
1.85 |
57 |
2.64 |
16 |
1.31 |
37 |
1.88 |
58 |
2.69 |
17 |
1.34 |
38 |
1.91 |
59 |
2.73 |
18 |
1.36 |
39 |
1.95 |
60 |
2.78 |
19 |
1.38 |
40 |
1.98 |
||
20 |
1.41 |
41 |
2.01 |
||
* Day of Calibration.
RUBY-FILL Expiration
Stop use of the RUBY-FILL Rubidium Rb 82 Generator once any one of the following Expiration Limits is reached:
- A total elution volume of 30 L has passed through the generator column, or
- Expiration date of the generator (60 days post-calibration), or
- An eluate Sr 82 level of 0.01 mcCi/mCi (kBq/MBq) Rb 82, or
- An eluate Sr 85 level of 0.1 mcCi/mCi (kBq/MBq) Rb 82.
RUBY-FILL Dose Delivery Limit
The maximum available activity (delivery limit) will decrease as the generator ages. Certain doses, including the maximum recommended dose [60 mCi (2220 MBq)], are not achievable for the entire shelf-life of the generator. Table 3 provides an estimate of the maximum available activity of Rubidium Rb 82 (Delivery Limit) as a function of generator age.
|
Generator Age (days) 2 |
Maximum Rubidium Dose (Delivery Limit) |
|
0 to 17 |
60 mCi (2220 MBq) |
|
24 |
50 mCi (1850 MBq) |
|
32 |
40 mCi (1480 MBq) |
|
42 |
30 mCi (1110 MBq) |
|
57 |
20 mCi ( 740 MBq) |
1Estimate is based on a 100 mCi (3700 MBq) Sr 82 generator at calibration.
2Generator age at which delivery limit is reached varies with generator activity at release. For example, an 85 mCi (3145 MBq) generator and a 115 mCi (4255 MBq) generator will reach a delivery limit < 60 mCi at ≥ 12 days and ≥ 23 days, respectively.
Radiation Dosimetry
The estimated radiation absorbed dose coefficients for Rb 82, Sr 82, and Sr 85 from an intravenous injection of rubidium Rb 82 chloride are shown in Table 4.
|
TABLE 4 |
|||
|
Organ |
82Rb1 (mcGy/MBq) |
82Sr2 (mcGy/kBq) |
85Sr2 (mcGy/kBq) |
|
Adrenals |
2.4 |
2.9 |
1.4 |
|
Bone surfaces |
0.42 |
29 |
2.7 |
|
Brain |
0.14 |
2.2 |
0.8 |
|
Breast |
0.19 |
1.9 |
0.5 |
|
Gallbladder wall |
0.72 |
2.3 |
0.8 |
|
Gastrointestinal tract |
|||
|
Esophagus3 |
1.5 |
2.1 |
0.6 |
|
Stomach wall |
0.83 |
2.1 |
0.6 |
|
Small intestine wall |
2.0 |
2.6 |
1.1 |
|
Colon wall |
1.1 |
9.7 |
1.2 |
|
(ULI wall) |
1.1 |
6.4 |
1.0 |
|
(LLI wall) |
1.1 |
14 |
1.4 |
|
Heart wall |
4.0 |
2.2 |
0.7 |
|
Kidneys |
9.3 |
2.5 |
0.7 |
|
Liver |
1.0 |
2.2 |
0.7 |
|
Lungs |
2.6 |
2.2 |
0.8 |
|
Muscles |
0.23 |
2.2 |
0.7 |
|
Ovaries |
0.50 |
2.8 |
1.2 |
|
Pancreas |
2.6 |
2.5 |
0.9 |
|
Red marrow |
0.38 |
25 |
2.7 |
|
Skin |
0.18 |
1.9 |
0.5 |
|
Spleen |
0.18 |
2.2 |
0.7 |
|
Testes |
0.26 |
2.0 |
0.5 |
|
Thymus |
1.5 |
2.1 |
0.6 |
|
Thyroid |
0.31 |
2.2 |
0.7 |
|
Urinary bladder wall |
0.18 |
5.9 |
0.8 |
|
Uterus |
1.0 |
2.5 |
0.9 |
|
Remaining organs |
0.31 |
- |
- |
|
Effective dose per unit activity |
1.1 mcSv/MBq |
6.3 mcSv/kBq |
1.1 mcSv/kBq |
|
1 Rb 82 doses are averages of rest and stress dosimetry data. To calculate organ doses (mcGy) from Rb 82, multiply the dose coefficient for each organ by the administered activity in MBq. 2 To calculate organ doses attributable to Sr 82 and Sr 85, multiply those dose coefficients by the respective strontium activities associated with the injection. 3The absorbed dose to the thymus is used as a substitute. |
|||
More about Ruby-Fill (rubidium chloride rb-82)
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

