Extraneal: Package Insert / Prescribing Info
Package insert / product label
Generic name: icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride
Dosage form: injection, solution
Drug class: Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on May 9, 2025.
Related/similar drugs
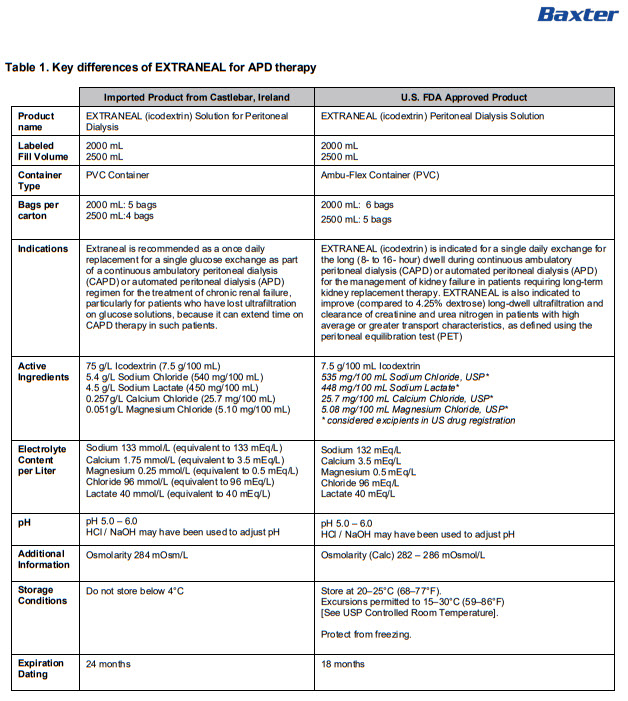
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
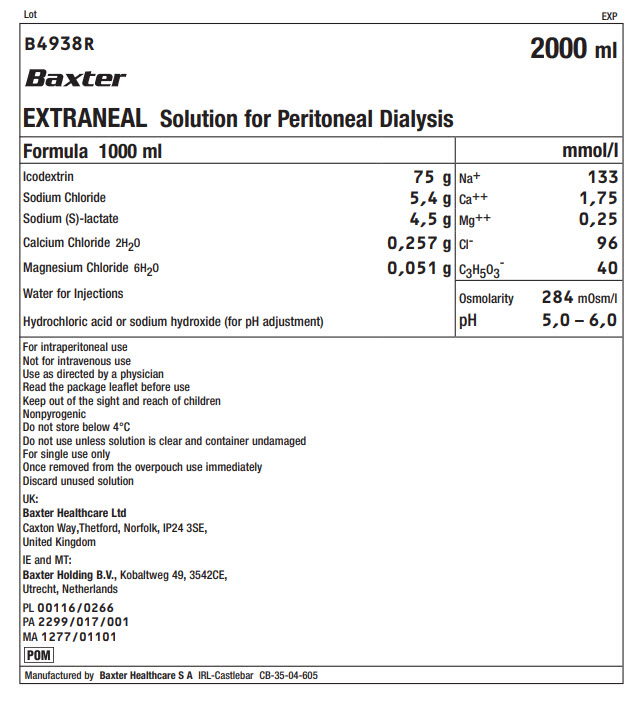
Lot EXP
B4938R 2000 ml
Baxter
EXTRANEAL Solution for Peritoneal Dialysis
|
Formula 1000 ml |
mmol/l |
||
|
Icodextrin |
75 g |
Na+ |
133 |
|
Sodium Chloride |
5,4 g |
Ca++ |
1,75 |
|
Sodium (S)-lactate |
4,5 g |
Mg++ |
0,25 |
|
Calcium Chloride 2H 20 |
0,257 g |
Clˉ |
96 |
|
Magnesium Chloride 6H 20 |
0,051 g |
C 3H 50 3ˉ |
40 |
|
Water for Injections |
Osmolarity |
284mOsm/l |
|
|
Hydrochloric acid or sodium hydroxide (for pH adjustment) |
pH |
5,0 – 6,0 |
|
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
UK:
Baxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE,
United Kingdom
IE and MT:
Baxter Holding B.V.,
Kobaltweg 49, 3542CE,
Utrecht, Netherlands
PL 00116/0266
PA 2299/017/001
MA 1277/01101
POM Symbol
Manufactured by Baxter Healthcare S A IRL-Castlebar CB-35-04-605
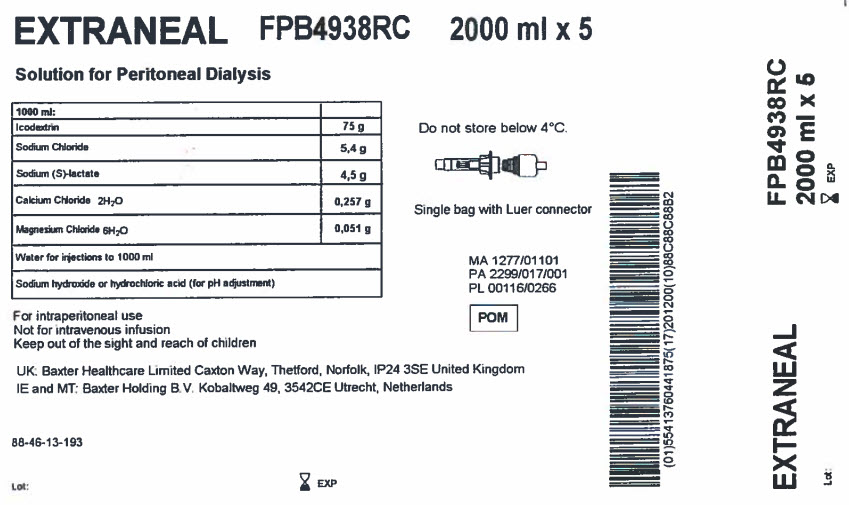
EXTRANEAL
FPB4938RC
2000 ml x 5
Solution for Peritoneal Dialysis
|
1000 ml |
|
|
Icodextrin |
75 g |
|
Sodium Chloride |
5,4 g |
|
Sodium (S)-lactate |
4,5 g |
|
Calcium Chloride 2H 20 |
0,257 g |
|
Magnesium Chloride 6H 20 |
0,051 g |
|
Water for injections to 1000 ml |
|
|
Sodium hydroxide or hydrochloric acid (for pH adjustment) |
|
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of children
UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V.
Kobaltweg 49, 3542CE, Utrecht, Netherlands
88-46-13-193
Lot: EXP
Do not store below 4°C
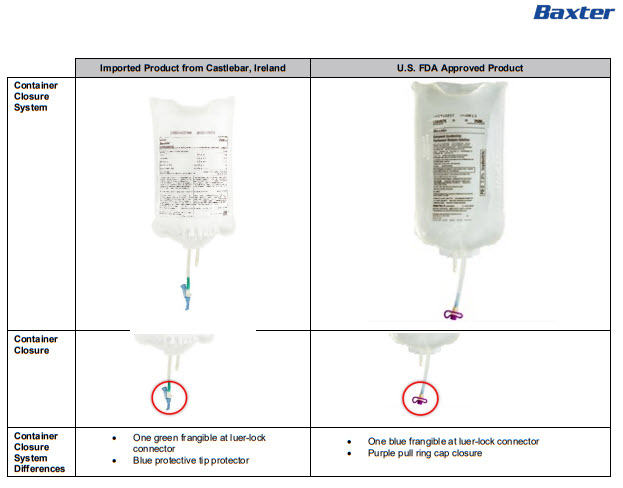
Single bag with Luer connector
MA 1277/01101
PA 2299/017/001
PL 00116/0266
POM Symbol
Barcode
(01)55413760441875(17)201200(10)88C88C88B2
EXTRANEAL
FPB4938RC
2000 ml x 5
Lot: EXP
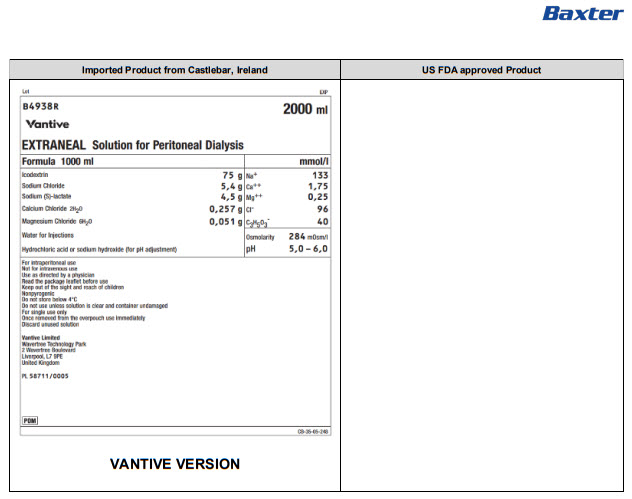
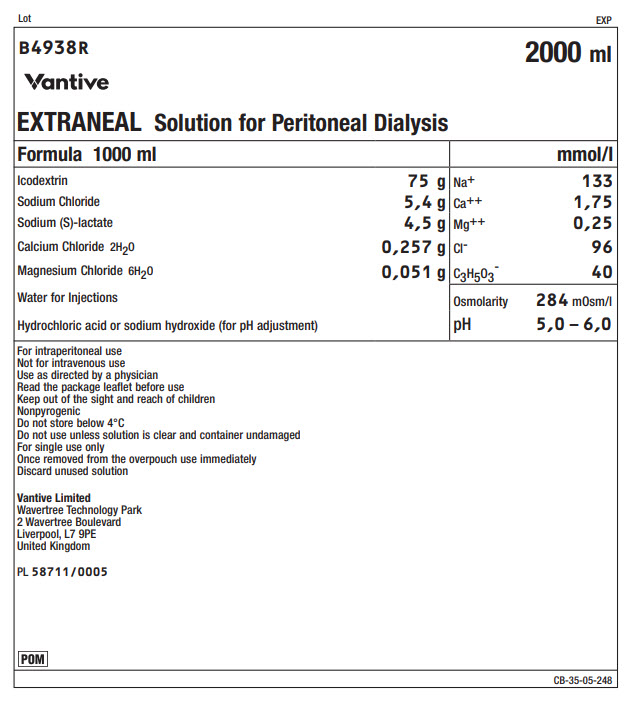
Lot EXP
B4938R 2000 ml
Vantive
EXTRANEAL Solution for Peritoneal Dialysis
|
Formula 1000 ml |
mmol/l |
||
|
Icodextrin |
75 g |
Na+ |
133 |
|
Sodium Chloride |
5,4 g |
Ca++ |
1,75 |
|
Sodium (S)-lactate |
4,5 g |
Mg++ |
0,25 |
|
Calcium Chloride 2H 20 |
0,257 g |
Clˉ |
96 |
|
Magnesium Chloride 6H 20 |
0,051 g |
C 3H 50 3ˉ |
40 |
|
Water for Injections |
Osmolarity |
284mOsm/l |
|
|
Hydrochloric acid or sodium hydroxide (for pH adjustment) |
pH |
5,0 – 6,0 |
|
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Vantive Limited
Wavertree Technology Park
2 Wavertree Boulevard
Liverpool, L7 9PE
United Kingdom
PL 58711/0005
POM Symbol
CB-35-05-248
EXTRANEAL
FPB4938RC
2000 ml x 5
Solution for Peritoneal Dialysis
|
1000 ml |
|
|
Icodextrin |
75 g |
|
Sodium Chloride |
5,4 g |
|
Sodium (S)-lactate |
4,5 g |
|
Calcium Chloride 2H 20 |
0,257 g |
|
Magnesium Chloride 6H 20 |
0,051 g |
|
Water for injections to 1000 ml |
|
|
Sodium hydroxide or hydrochloric acid (for pH adjustment) |
|
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of children
Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
88-46-14-004
Lot: EXP
UK Only
Do not store below 4°C
Single bag with Luer connector
PL 58711/0005
POM Symbol
Barcode
(01)57332414202786(17)201200(10)88C88C88B2
EXTRANEAL
FPB4938RC
2000 ml x 5
Lot: EXP
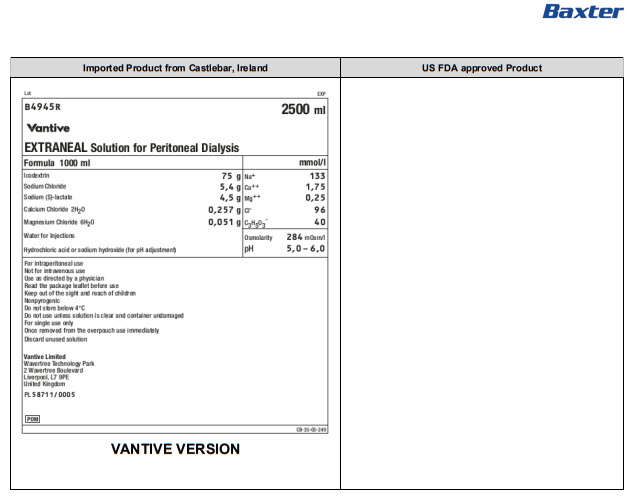
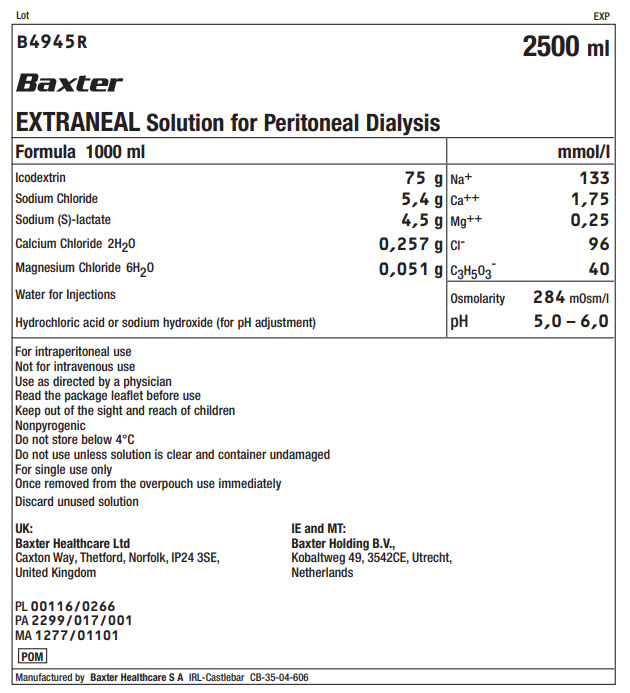
Lot EXP
B4945R 2500 ml
Baxter
EXTRANEAL Solution for Peritoneal Dialysis
|
Formula 1000 ml |
mmol/l |
||
|
Icodextrin |
75 g |
Na+ |
133 |
|
Sodium Chloride |
5,4 g |
Ca++ |
1,75 |
|
Sodium (S)-lactate |
4,5 g |
Mg++ |
0,25 |
|
Calcium Chloride 2H 20 |
0,257 g |
Clˉ |
96 |
|
Magnesium Chloride 6H 20 |
0,051 g |
C 3H 50 3ˉ |
40 |
|
Water for Injections |
Osmolarity |
284mOsm/l |
|
|
Hydrochloric acid or sodium hydroxide (for pH adjustment) |
pH |
5,0 – 6,0 |
|
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
UK:
Baxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE,
United Kingdom
IE and MT:
Baxter Holding B.V.,
Kobaltweg 49, 3542CE, Utrecht,
Netherlands
PL 00116/0266
PA 2299/017/001
MA 1277/01101
POM Symbol
Manufactured by Baxter Healthcare S A IRL-Castlebar CB-35-04-606
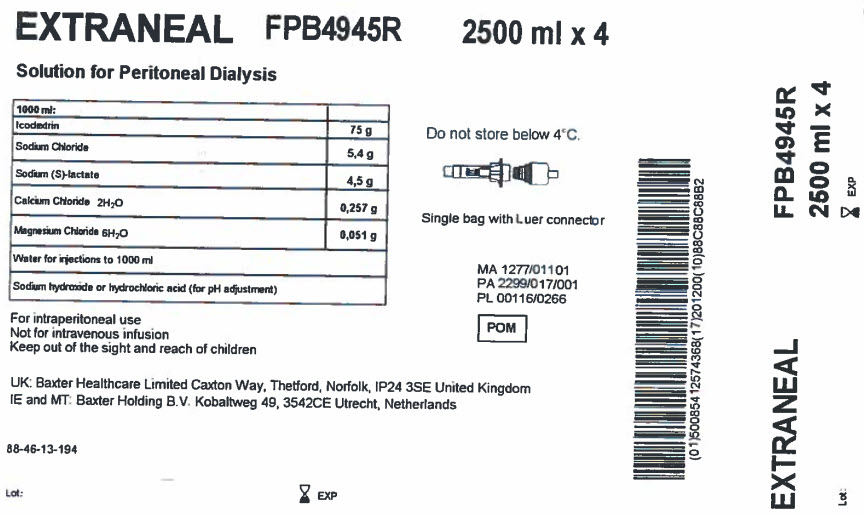
EXTRANEAL
FPB4945R
2500 ml x 4
Solution for Peritoneal Dialysis
|
1000 ml |
|
|
Icodextrin |
75 g |
|
Sodium Chloride |
5,4 g |
|
Sodium (S)-lactate |
4,5 g |
|
Calcium Chloride 2H 20 |
0,257 g |
|
Magnesium Chloride 6H 20 |
0,051 g |
|
Water for injections to 1000 ml |
|
|
Sodium hydroxide or hydrochloric acid (for pH adjustment) |
|
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of children
UK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 3542CE, Utrecht, Netherlands
88-46-13-194
Lot: EXP
Do not store below 4°C
Single bag with Luer connector
MA 1277/01101
PA 2299/017/001
PL 00116/0266
POM Symbol
Barcode
(01)50085412574368(17)201200(10)88C88C88B2
EXTRANEAL
FPB4945R
2500 ml x 4
Lot: EXP
Lot EXP
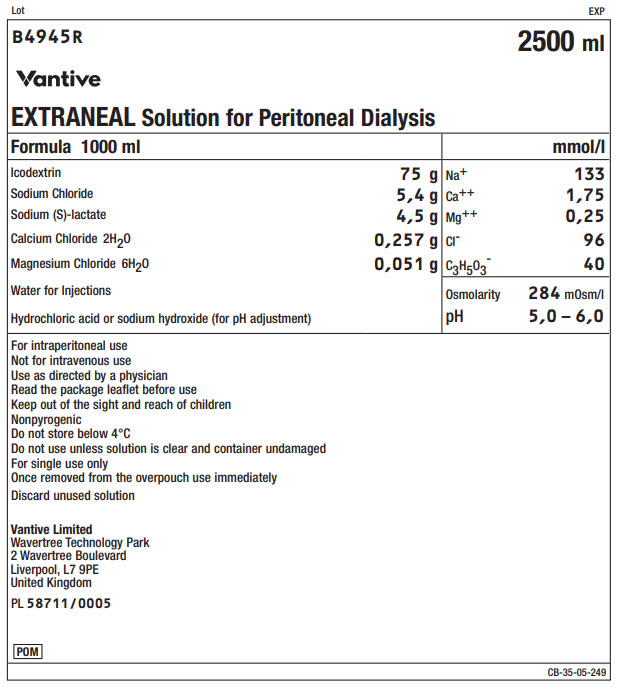
B4945R 2500 ml
Vantive
EXTRANEAL Solution for Peritoneal Dialysis
|
Formula 1000 ml |
mmol/l |
||
|
Icodextrin |
75 g |
Na+ |
133 |
|
Sodium Chloride |
5,4 g |
Ca++ |
1,75 |
|
Sodium (S)-lactate |
4,5 g |
Mg++ |
0,25 |
|
Calcium Chloride 2H 20 |
0,257 g |
Clˉ |
96 |
|
Magnesium Chloride 6H 20 |
0,051 g |
C 3H 50 3ˉ |
40 |
|
Water for Injections |
Osmolarity |
284mOsm/l |
|
|
Hydrochloric acid or sodium hydroxide (for pH adjustment) |
pH |
5,0 – 6,0 |
|
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solution
Vantive Limited
Wavertree Technology Park
2 Wavertree Boulevard
Liverpool, L7 9PE
United Kingdom
PL 58711/0005
POM Symbol
CB-35-05-249
EXTRANEAL
FPB4945R
2500 ml x 4
Solution for Peritoneal Dialysis
|
1000 ml |
|
|
Icodextrin |
75 g |
|
Sodium Chloride |
5,4 g |
|
Sodium (S)-lactate |
4,5 g |
|
Calcium Chloride 2H 20 |
0,257 g |
|
Magnesium Chloride 6H 20 |
0,051 g |
|
Water for injections to 1000 ml |
|
|
Sodium hydroxide or hydrochloric acid (for pH adjustment) |
|
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of children
Vantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
88-46-14-005
Lot: EXP
UK Only
Do not store below 4°C
Single bag with Luer connector
PL 58711/0005
POM Symbol
Barcode
(01)57332414203240(17)201200(10)88C88C88B2
EXTRANEAL
FPB4945R
2500 ml x 4
Lot: EXP
| EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Vantive US Healthcare LLC (119181963) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare S.A. | 988899845 | analysis(0941-0681, 0941-0719) , label(0941-0681, 0941-0719) , manufacture(0941-0681, 0941-0719) , pack(0941-0681, 0941-0719) , sterilize(0941-0681, 0941-0719) , api manufacture(0941-0681, 0941-0719) | |
More about Extraneal (lvp solution)
Patient resources
Professional resources
Other brands
Lactated Ringers Injection, Isolyte S, Normosol-R, Delflex, ... +2 more