Breyanzi Dosage
Generic name: LISOCABTAGENE MARALEUCEL 500000001 in 5mL
Dosage form: intravenous suspension
Drug class: Miscellaneous antineoplastics
Medically reviewed by Drugs.com. Last updated on Mar 14, 2024.
For autologous use only. For intravenous use only.
Dose
See the respective Certificate of Release for Infusion (RFI Certificate) for each component, for the actual cell counts and volumes to be infused [see Dosage and Administration (2.2) and Dosage Forms and Strengths (3)].
A single dose of BREYANZI contains CAR-positive viable T cells (consisting of 1:1 CAR-positive viable T cells of the CD8 and CD4 components), with each component supplied separately in one to four single-dose vials. See Table 1 for dose range per indication.
Table 1: Dose Range
|
Indication |
BREYANZI dose range |
|
LBCL after two or more lines of therapy (1.1) |
50 to 110 × 106 CAR-positive viable T cells |
|
LBCL after one line of therapy (1.1) |
90 to 110 × 106 CAR-positive viable T cells |
|
CLL or SLL (1.2) |
Administration
BREYANZI is for autologous use only. The patient’s identity must match the patient identifiers on the BREYANZI cartons, vials and syringe labels. Do not infuse BREYANZI if the information on the patient-specific labels does not match the intended patient.
Preparing the Patient for BREYANZI
Confirm the availability of BREYANZI before starting lymphodepleting chemotherapy.
Pretreatment
Administer the lymphodepleting chemotherapy regimen before infusion of BREYANZI: fludarabine 30 mg/m2/day intravenously (IV), and cyclophosphamide 300 mg/m2/day IV for 3 days. See the prescribing information for fludarabine and cyclophosphamide for information on dose adjustment in renal impairment.
Infuse BREYANZI 2 to 7 days after completion of lymphodepleting chemotherapy.
Delay the infusion of BREYANZI if the patient has unresolved serious adverse events from preceding chemotherapies, active uncontrolled infection, or active graft-versus-host disease (GVHD).
Premedication
To minimize the risk of infusion reactions, premedicate the patient with acetaminophen (650 mg orally) and diphenhydramine (25-50 mg, IV or orally), or another H1-antihistamine, 30 to 60 minutes prior to treatment with BREYANZI.
Avoid prophylactic use of systemic corticosteroids, as they may interfere with the activity of BREYANZI.
Receipt of BREYANZI
- •
- BREYANZI is shipped directly to the cell-associated lab or clinical pharmacy associated with the infusion center in the vapor phase of a liquid nitrogen shipper.
- •
- Confirm the patient’s identity with the patient identifiers on the shipper.
- •
- If the patient is not expected to be ready for administration before the shipper expires and the infusion site is qualified for onsite storage, transfer BREYANZI to onsite vapor phase of liquid nitrogen storage prior to preparation.
- •
- If the patient is not expected to be ready for administration before the shipper expires and the infusion site is not qualified for onsite storage, contact Bristol-Myers Squibb at 1-888-805-4555 to arrange for return shipment.
Preparing BREYANZI
Before thawing the vials
- •
- Confirm the patient’s identity with the patient identifiers on the RFI Certificate.
- •
- Read the RFI Certificate (affixed inside the shipper) for information on the number of syringes you will need to administer the CD8 and CD4 components (syringe labels are provided with the RFI Certificate). There is a separate RFI Certificate for each cell component.
- •
- Confirm tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- •
- Confirm the infusion time in advance and adjust the start time of BREYANZI thaw such that it will be available for infusion when the patient is ready.
Thawing the vials
- 1.
- Confirm the patient’s identity with the patient identifiers on the outer carton and on the syringe labels.
Once the vials of CAR-positive viable T cells (CD8 component and CD4 component) are removed from frozen storage, the thaw must be carried to completion and the cells administered within 2 hours. - 2.
- Remove the CD8 component carton and CD4 component carton from the outer carton.
- 3.
- Confirm the patient’s identity with the patient identifiers on the inner carton.
- 4.
- Open each inner carton and visually inspect the vial(s) for damage. If the vials are damaged, contact Bristol-Myers Squibb at 1-888-805-4555.
- 5.
- Confirm the patient’s identity with the patient identifiers on the vials.
- 6.
- Carefully remove the vials from the cartons, place vials on a protective barrier pad, and thaw at room temperature until there is no visible ice in the vials. Thaw all of the vials at the same time.
Keep the CD8 and CD4 components separate.
Dose preparation
- •
- Prepare BREYANZI using sterile technique.
- •
- Based on the concentration of CAR-positive viable T cells for each component, more than one vial of each of the CD8 and CD4 components may be required to complete a dose. A separate syringe should be prepared for each CD8 or CD4 component vial received.
Note: The volume to be drawn up and infused may differ for each component as indicated on the RFI Certificate. Do NOT draw up excess volume into the syringe. - •
- Each vial contains 5 mL with a total extractable volume of 4.6 mL of CD8 or CD4 component T cells. The RFI Certificate for each component indicates the volume (mL) of cells to be drawn up into each syringe. Use the smallest Luer-lock tip syringe necessary (1, 3, or 5 mL) to draw up the specified volume from each vial. A 5 mL syringe should not be used for volumes less than 3 mL.
- 7.
- Prepare the syringe(s) of the CD8 component first. Affix the CD8 syringe labels to the syringe(s) prior to pulling the required volume into the syringe(s).
Note: It is important to confirm that the volume drawn up for each component matches the volume specified in the respective RFI Certificate. Do NOT draw up excess volume into the syringe.
Withdrawal of the required volume of cells from each vial into a separate syringe should be carried out using the following instructions:
- 8.
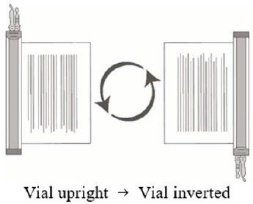
- Hold the thawed vial(s) upright and gently invert the vial(s) 5 times to mix the cell product. If any clumping is apparent, continue to invert the vial(s) until clumps have dispersed and cells appear to be evenly resuspended.
- 9.
- Visually inspect the thawed vial(s) for damage or leaks. Do not use if the vial is damaged or if the clumps do not disperse; contact Bristol-Myers Squibb at 1-888-805-4555. The liquid in the vials should be slightly opaque to opaque, colorless to yellow or brownish-yellow.
- 10.
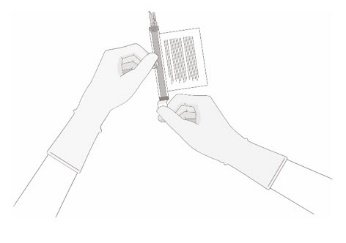
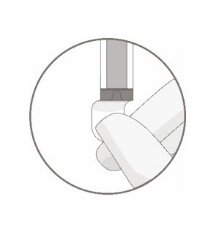
- Remove the polyaluminum cover (if present) from the bottom of the vial and swab the septum with an alcohol wipe. Allow to air dry before proceeding.
NOTE: The absence of the polyaluminum cover does not impact the sterility of the vial.
 |
 |
- 11.
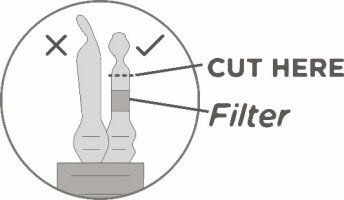
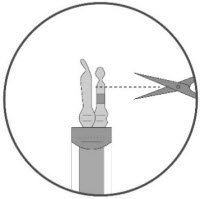
- Keeping the vial(s) upright, cut the seal on the tubing line on the top of the vial immediately above the filter to open the air vent on the vial.
NOTE: Be careful to select the correct tubing line with the filter. Cut ONLY the tubing with a filter.
 |
 |
- 12.
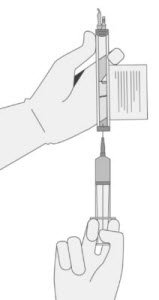
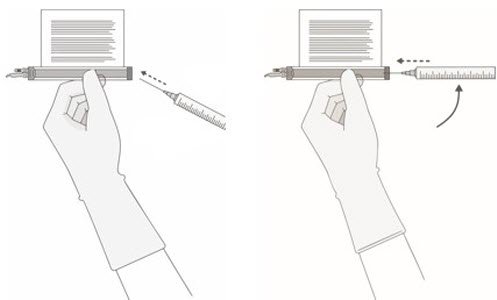
- Hold a 20-gauge, 1-1 ½ inch needle, with the opening of the needle tip away from the retrieval port septum.
- a.
- Insert the needle into the septum at a 45°- 60° angle to puncture the retrieval port septum.
- b.
- Increase the angle of the needle gradually as the needle enters the vial.
|
a |
b |
 |
|
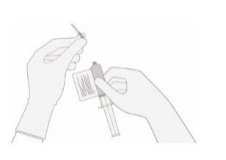
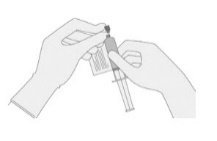
- 13.
- WITHOUT drawing air into the syringe, slowly withdraw the target volume (as specified in the RFI Certificate). Carefully inspect the syringe for signs of debris prior to proceeding. If there is debris, contact Bristol-Myers Squibb at 1-888-805-4555.
- 14.
- Verify that the volume of CD8/CD4 component matches the volume specified for the relevant component in the RFI Certificate.
Once the volume is verified, remove the syringe/needle from the vial, carefully detach the needle from the syringe and cap the syringe.
 |
 |
 |
- 15.
- Continue to keep the vial horizontal and return it to the carton to avoid leaking from the vial.
- 16.
- Dispose of any unused portion of BREYANZI (according to local biosafety guidelines).
- 17.
- Repeat the process steps 7-16 for the CD4 Component.
- 18.
- Transport the labeled CD8 and CD4 syringes to the bedside by placing with protective barrier pad inside an insulated room temperature container.
BREYANZI Administration
- •
- Do NOT use a leukodepleting filter.
- •
- Ensure tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- •
- Confirm the patient’s identity matches the patient identifiers on the syringe label.
- •
- Once BREYANZI has been drawn into syringes, proceed with administration as soon as possible. The total time from removal from frozen storage to patient administration should not exceed 2 hours as indicated by the time entered on the syringe label.
- 1.
- Use intravenous normal saline to flush all the infusion tubing prior to and after each CD8 or CD4 component administration.
- 2.
- Administer the entire volume of the CD8 component intravenously at an infusion rate of approximately 0.5 mL/minute, using the closest port or Y-arm.
NOTE: The time for infusion will vary but will usually be less than 15 minutes for each component. - 3.
- If more than one syringe is required for a full cell dose of the CD8 component, administer the volume in each syringe consecutively without any time between administering the contents of the syringes (unless there is a clinical reason (e.g., infusion reaction) to hold the dose).
- 4.
- After the CD8 component has been administered, flush the tubing with normal saline, using enough volume to clear the tubing and the length of the IV catheter.
- 5.
- Administer the CD4 component second, immediately after administration of the CD8 component is complete, using steps 1-4, as described for the CD8 component. Following administration of the CD4 component, flush the tubing with normal saline, using enough volume to clear the tubing and the length of the IV catheter.
BREYANZI contains human blood cells that are genetically modified with replication-incompetent, self-inactivating lentiviral vector. Follow universal precautions and local biosafety guidelines applicable for the handling and disposal, to avoid potential transmission of infectious diseases.
Monitoring
- •
- Administer BREYANZI at a REMS-certified healthcare facility.
- •
- Monitor patients daily for at least 7 days following BREYANZI infusion at a REMS-certified healthcare facility for signs and symptoms of CRS and neurologic toxicities.
- •
- Instruct patients to remain within proximity of the certified healthcare facility for at least 4 weeks following infusion.
- •
- Instruct patients to refrain from driving or hazardous activities for at least 8 weeks following infusion.
Management of Severe Adverse Reactions
Cytokine Release Syndrome
Identify cytokine release syndrome (CRS) based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 2. Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive-care supportive therapy.
If concurrent neurologic toxicity is suspected during CRS, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 2 and 3
- •
- Tocilizumab according to the CRS grade in Table 2
- •
- Antiseizure medication according to the neurologic toxicity in Table 3
| a Lee criteria for grading CRS (Lee et al, 2014). | ||
| b If corticosteroids are initiated, continue corticosteroids for at least 3 doses or until complete resolution of symptoms, and consider corticosteroid taper. | ||
|
CRS Gradea |
Tocilizumab |
Corticosteroidsb |
|
Grade 1 Fever |
If less than 72 hours after infusion, consider tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). If 72 hours or more after infusion, treat symptomatically. |
If less than 72 hours after infusion, consider dexamethasone 10 mg IV every 24 hours. If 72 hours or more after infusion, treat symptomatically. |
|
Grade 2 Symptoms require and respond to moderate intervention. Oxygen requirement less than 40% FiO2, or hypotension responsive to fluids or low dose of one vasopressor, or Grade 2 organ toxicity. |
Administer tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). Repeat tocilizumab every 8 hours as needed if not responsive to intravenous fluids or increasing supplemental oxygen. |
If less than 72 hours after infusion, administer dexamethasone 10 mg IV every 12‑24 hours. If 72 hours or more after infusion, consider dexamethasone 10 mg IV every 12‑24 hours. |
|
If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (10‑20 mg IV every 6 to 12 hours). If no improvement or continued rapid progression, maximize dexamethasone, switch to high-dose methylprednisolone 2 mg/kg if needed. After 2 doses of tocilizumab, consider alternative immunosuppressants. Do not exceed 3 doses tocilizumab in 24 hours, or 4 doses in total. |
||
|
Grade 3 Symptoms require and respond to aggressive intervention. Oxygen requirement greater than or equal to 40% FiO2, or hypotension requiring high-dose or multiple vasopressors, or Grade 3 organ toxicity, or Grade 4 transaminitis. |
Per Grade 2. |
Administer dexamethasone 10 mg IV every 12 hours. |
|
If no improvement within 24 hours or rapid progression of CRS, repeat tocilizumab and escalate dose and frequency of dexamethasone (10-20 mg IV every 6 to 12 hours). If no improvement or continued rapid progression, maximize dexamethasone, switch to high-dose methylprednisolone 2 mg/kg if needed. After 2 doses of tocilizumab, consider alternative immunosuppressants. Do not exceed 3 doses tocilizumab in 24 hours, or 4 doses in total. |
||
|
Grade 4 Life-threatening symptoms Requirements for ventilator support or continuous veno‑venous hemodialysis (CVVHD) or Grade 4 organ toxicity (excluding transaminitis). |
Per Grade 2. |
Administer dexamethasone 20 mg IV every 6 hours. |
|
If no improvement within 24 hours or rapid progression of CRS, escalate tocilizumab and corticosteroid use. If no improvement or continued rapid progression, maximize dexamethasone, switch to high-dose methylprednisolone 2 mg/kg if needed. After 2 doses of tocilizumab, consider alternative immunosuppressants. Do not exceed 3 doses tocilizumab in 24 hours, or 4 doses in total. |
||
Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicities (Table 3). Rule out other causes of neurologic symptoms. Provide intensive care supportive therapy for severe or life-threatening neurologic toxicities. If neurologic toxicity is suspected, manage according to the recommendations in Table 3.
If concurrent CRS is suspected during neurologic toxicity, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 2 and 3
- •
- Tocilizumab according to the CRS grade in Table 2
- •
- Antiseizure medication according to the neurologic toxicity in Table 3
| NT Gradea | Corticosteroids and Antiseizure Medication |
|---|---|
| a NCI CTCAE criteria for grading neurologic toxicities, version 4.03. | |
|
Grade 1 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 2 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 3 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 4 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
More about Breyanzi (lisocabtagene maraleucel)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Latest FDA alerts (2)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous antineoplastics
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.