Alphanine SD Dosage
Generic name: COAGULATION FACTOR IX HUMAN 500[iU] in 10mL; water 1mL in 1mL
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Jan 18, 2024.
For adult usage:
AlphaNine SD should be administered intravenously promptly following reconstitution. Administration of AlphaNine SD within three hours after reconstitution is recommended to avoid the potential ill effect of any inadvertent bacterial contamination occurring during reconstitution. Discard any unused contents into the appropriate safety container.
Each vial of AlphaNine SD is labeled with the total units expressed as International Units (IU) of Factor IX, which is referenced to the WHO International Standard. One unit approximates the activity in one mL of pooled normal human plasma.
The amount of AlphaNine SD required to establish hemostasis will vary with each patient and depend upon the circumstances. The following formula may be used as a guide in determining the number of units to be administered.16
In clinical practice there is variability between patients and their clinical response. Therefore, the Factor IX level of each patient should be monitored frequently during replacement therapy.
For pediatric usage: See PRECAUTIONS
| Type of Hemorrhage or Surgical Procedure | Examples | Treatment Guidelines |
| Minor Hemorrhages | Bruises, cuts or scrapes, uncomplicated joint hemorrhage | FIX levels should be brought to at least 20-30% (20-30 IU FIX/kg/twice daily) until hemorrhage stops and healing has been achieved (1-2 days).17,18,19 |
| Moderate Hemorrhages | Nose bleeds, mouth and gum bleeds, dental extractions, hematuria | FIX levels should be brought to 25-50% (25-50 IU FIX/kg/twice daily) until healing has been achieved (2-7 days, on average).17,18,19,20,21 |
| Major Hemorrhages | Joint and muscle hemorrhages (especially in the large muscles), major trauma, hematuria, intracranial and intraperitoneal bleeding | FIX levels should be brought to 50% for at least 3-5 days (30-50 IU FIX/kg/twice daily). Following this treatment period, FIX levels should be maintained at 20% (20 IU FIX/kg/twice daily) until healing has been achieved. Major hemorrhages may require treatment for up to 10 days.17,18,19,20,21 |
| Surgery | Prior to surgery, FIX should be brought to 50-100% of normal (50-100 IU FIX/kg/twice daily). For the next 7 to 10 days, or until healing has been achieved, the patient should be maintained at 50-100% FIX levels (50-100 IU FIX/kg/twice daily).17,18,19,20,21 |
Dosing requirements and frequency of dosing is calculated on the basis of an initial response of 1% FIX increase achieved per IU of FIX infused per kg body weight and an average half-life for FIX of 18 hours. If dosing studies have revealed that a particular patient exhibits a lower response, the dose should be adjusted accordingly.
For pediatric usage: See PRECAUTIONS
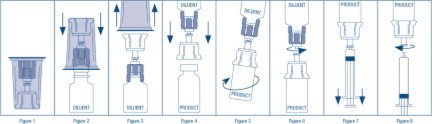
RECONSTITUTION
Use Aseptic Technique
- Warm diluent (Sterile Water for Injection, USP) and concentrate (AlphaNine SD) to at least room temperature (but not above 37 °C).
- Remove the plastic flip off cap from the diluent vial.
- Gently swab the exposed stopper surface with a cleansing agent such as alcohol trying to avoid leaving any excess cleansing agent on the stopper.
- Open the Mix2Vial® package by peeling away the lid (Figure 1). Leave the Mix2Vial in the clear outer packaging.
- Place the diluent vial upright on an even surface and hold the vial tight and pick up the Mix2Vial in its clear outer packaging. Holding the diluent vial securely, push the blue end of the Mix2Vial vertically down through the diluent vial stopper (Figure 2).
- While holding onto the diluent vial, carefully remove the clear outer packaging from the Mix2Vial set, ensuring the Mix2Vial remains attached to the diluent vial (Figure 3).
- Place the product vial upright on an even surface, invert the diluent vial with the Mix2Vial attached.
- While holding the product vial securely on a flat surface, push the clear end of the Mix2Vial set vertically down through the product vial stopper (Figure 4). The diluent will automatically transfer out of its vial into the product vial. (NOTE: If the Mix2Vial is connected at an angle, the vacuum may be released from the product vial and the diluent will not transfer into the product vial.)
- With the diluent and product vials still attached to the Mix2Vial, gently swirl the product vial to ensure the product is fully dissolved (Figure 5). Reconstitution requires less than 5 minutes. Do not shake the vial.
- Disconnect the Mix2Vial into two separate pieces (Figure 6) by holding each vial adapter and twisting counterclockwise. After separating, discard the diluent vial with the blue end of the Mix2Vial.
- Draw air into an empty, sterile syringe. Keeping the product vial upright with the clear end of the Mix2Vial attached, screw the disposable syringe onto the luer lock portion of the Mix2Vial device by pressing and twisting clockwise. Inject air into the product vial.
- While keeping the syringe plunger depressed, invert the system upside down and draw the reconstituted product into the syringe by pulling the plunger back slowly (Figure 7).
- When the reconstituted product has been transferred into the syringe, firmly hold the barrel of the syringe and the clear vial adapter (keeping the syringe plunger facing down) and unscrew the syringe from the Mix2Vial (Figure 8). Hold the syringe upright and push the plunger until no air is left in the syringe. Attach the syringe to a venipuncture set.
- NOTE: If the same patient is to receive more than one vial of concentrate, the contents of two vials may be drawn into the same syringe through a separate unused Mix2Vial set before attaching to the venipuncture set.
- Use the prepared drug as soon as possible within three hours after reconstitution.
- After reconstitution, parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. When reconstitution procedure is strictly followed, a few small particles may occasionally remain. The Mix2Vial set will remove particles and the labeled potency will not be reduced.
- Discard all administration equipment after use into the appropriate safety container. Do not reuse.
More about Alphanine SD (coagulation factor ix)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- During pregnancy
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
BeneFix, Idelvion, Alprolix, Ixinity, ... +3 more
Professional resources
- Alphanine SD prescribing information
- Factor IX (Recombinant), albumin fusion protein (AHFS Monograph)
Other brands
BeneFix, Idelvion, Alprolix, Ixinity, ... +3 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.