Akovaz Dosage
Generic name: EPHEDRINE SULFATE 50mg in 1mL
Dosage form: injection
Drug classes: Decongestants, Vasopressors
Medically reviewed by Drugs.com. Last updated on Nov 17, 2023.
General Dosage and Administration Instructions
AKOVAZ (ephedrine sulfate injection), 50 mg/mL must be diluted before administration as an intravenous bolus to achieve the desired concentration. Dilute with normal saline or 5% dextrose in water.
AKOVAZ (ephedrine sulfate injection), 25 mg/5 mL (5 mg/mL) in a prefilled syringe, is a premixed formulation. Do not dilute prior to use. The single-dose prefilled syringe is intended for use in one patient during one surgical procedure. Discard any unused portion.
Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Dosing for the Treatment of Clinically Important Hypotension in the Setting of Anesthesia
AKOVAZ should be administered by trained healthcare providers.
The recommended dosages for the treatment of clinically important hypotension in the setting of anesthesia is an initial dose of 5 mg to 10 mg administered by intravenous bolus. Administer additional boluses as needed, not to exceed a total dosage of 50 mg.
- •
- Adjust dosage according to the blood pressure goal (i.e., titrate to effect).
Prepare a 5 mg/mL Solution for Bolus Intravenous Administration
For bolus intravenous administration, prepare a solution containing a final concentration of 5 mg/mL of AKOVAZ (ephedrine sulfate injection):
- •
- Withdraw 50 mg (1 mL of 50 mg/mL) of AKOVAZ (ephedrine sulfate injection) and dilute with 9 mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection.
- •
- Withdraw an appropriate dose of the 5 mg/mL solution prior to bolus intravenous administration.
Instructions for Use of Prefilled Syringe
1. Perform visual inspection on the syringe by verifying:
- •
- Absence of syringe damage
- •
- Absence of external particles
- •
- Absence of internal particles
- •
- Proper drug color
- •
- Drug name
- •
- Drug strength
- •
- Fill volume
- •
- Route of administration
- •
- Expiration date to be sure the drug has not expired
2. Do not remove tamper evident seal. Push plunger rod slightly in to break the stopper loose while tip cap is still on.
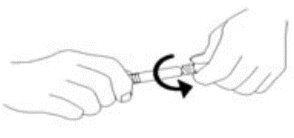
3. Remove tip cap and tamper evident seal by twisting off. (See Figure 1):
Figure 1.
4. Discard the tip cap.
5. Expel air bubble.
6. Adjust dose into sterile material (if applicable).
7. Connect the syringe to an appropriate intravenous connection.
- •
- Before injection, ensure that the syringe is securely attached to the needle or needleless luer access device (NLAD).
8. Depress plunger rod to deliver medication. Ensure that pressure is maintained on the plunger rod during the entire administration.
9. Remove syringe from NLAD (if applicable) and discard into appropriate receptacle.
- •
- To prevent needle stick injuries, do not recap needle when needle is connected to syringe.
NOTE: All steps must be done sequentially
- •
- Do not re-sterilize syringe
- •
- Do not use this product on a sterile field
- •
- Do not introduce any other fluid into the syringe at any time
- •
- This product is for single dose only
More about Akovaz (ephedrine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- Generic availability
- FDA approval history
- Drug class: decongestants
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.