Adakveo Injection Dosage
Generic name: CRIZANLIZUMAB 10mg in 1mL
Dosage form: injection
Drug class: Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Apr 18, 2023.
2.1 Recommended Dosage
Administer ADAKVEO 5 mg/kg by intravenous infusion over a period of 30 minutes at Week 0, Week 2, and every 4 weeks thereafter.
If a dose is missed, administer ADAKVEO as soon as possible.
If ADAKVEO is administered within 2 weeks after the missed dose, continue dosing according to the patient's original schedule.
If ADAKVEO is administered more than 2 weeks after the missed dose, continue dosing every 4 weeks thereafter.
ADAKVEO may be given with or without hydroxyurea.
2.2 Preparation and Administration
ADAKVEO should be prepared and administered by a healthcare professional.
Preparation
- Use aseptic technique to prepare the solution for infusion.
- Calculate the dose (mg) and the total volume (mL) of ADAKVEO solution required, and the number of ADAKVEO vials needed based on the patient’s actual body weight.
- Prepare 5 mg of ADAKVEO per kg of actual body weight.
- Calculate the volume of ADAKVEO to be used according to the following equation:
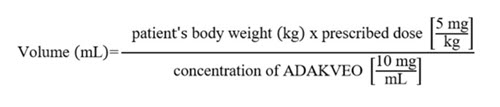
Dilution
Dilute ADAKVEO in 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP to a total volume of 100 mL for intravenous infusion as follows:
- Obtain the number of vials required. One vial is needed for every 10 mL of ADAKVEO.
- Bring vials to room temperature for a maximum of 4 hours prior to the start of preparation (piercing the first vial).
- Visually inspect the vials.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- ADAKVEO is clear to opalescent, colorless or may have a slightly brownish-yellow tint.
- Do not use if particles are present in the solution.
- Obtain a 100 mL 0.9% Sodium Chloride Injection or 5% Dextrose Injection infusion bag/container.
- Infusion bags/containers must be made of either polyvinyl chloride (PVC), polyethylene (PE), or polypropylene (PP).
- Remove a volume of 0.9% Sodium Chloride Injection or 5% Dextrose Injection from the infusion bag/container that is equal to the required volume of ADAKVEO solution.
- Withdraw the necessary amount of ADAKVEO solution and dilute by adding to the infusion bag/container containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection.
- The volume of ADAKVEO added to the infusion bag/container should not exceed 96 mL.
- Gently invert the infusion bag to mix the diluted solution. DO NOT SHAKE.
- Single-dose vials. Discard unused portion.
Storage Conditions of the Diluted Solution
Administer ADAKVEO diluted solution as soon as possible. If not administered immediately, store the prepared solution either:
- At room temperature up to 25°C (77°F) for no more than 4.5 hours from the start of the preparation (piercing the first vial) to the completion of infusion.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours, from the start of the time of the preparation (piercing the first vial) to the completion of infusion. This includes the storage of the diluted solution and the time to warm up to room temperature. Protect the diluted solution from light during storage under refrigeration.
Administration
- Administer ADAKVEO diluted solution by intravenous infusion over a period of 30 minutes through an intravenous line, which must contain a sterile, nonpyrogenic 0.2-micron inline filter.
- No incompatibilities have been observed between ADAKVEO and infusion sets composed of PVC, polyethylene (PE-lined PVC), polyurethane (PU), and in-line filter membranes composed of polyethersulfone (PES, neutral and positively charged), positively charged polyamide (PA), and polysulphone (PSU).
- Do not mix or coadminister with other drugs through the same intravenous line.
- After administration of ADAKVEO, flush the line with at least 25 mL of 0.9% Sodium Chloride or 5% Dextrose Injection.
- Dispose of any unused product or waste material in accordance with local requirements.
2.3 Management of Infusion-Related Reactions
No dose reductions are recommended. Management for infusion-related reactions for ADAKVEO is described in Table 1.
| aExercise caution with the use of corticosteroids in patients with sickle cell disease unless clinically indicated (e.g., treatment of anaphylaxis). | |
| Severity of Adverse Reaction | Recommendation |
| Mild to moderate infusion-related reactions |
|
| Severe infusion-related reactions |
|
Frequently asked questions
More about Adakveo (crizanlizumab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
