Cytoxan Prescribing Information
Package insert / product label
Generic name: cyclophosphamide
Dosage forms: injection, powder, for solution, tablets
Drug class: Alkylating agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
The Cytoxan brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Cytoxan Description
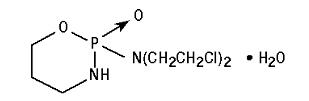
CYTOXAN® (cyclophosphamide for injection, USP) is a sterile white powder containing cyclophosphamide monohydrate. CYTOXAN Tablets (cyclophosphamide tablets, USP) are for oral use and contain 25 mg or 50 mg cyclophosphamide (anhydrous). Inactive ingredients in CYTOXAN Tablets are: acacia, FD&C Blue No. 1, D&C Yellow No. 10 Aluminum Lake, lactose, magnesium stearate, starch, stearic acid and talc. Cyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. Cyclophosphamide is a white crystalline powder with the molecular formula C7H15Cl2N2O2P•H2O and a molecular weight of 279.1. The chemical name for cyclophosphamide is 2-[bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide monohydrate. Cyclophosphamide is soluble in water, saline, or ethanol and has the following structural formula:
Cytoxan - Clinical Pharmacology
Cyclophosphamide is biotransformed principally in the liver to active alkylating metabolites by a mixed function microsomal oxidase system. These metabolites interfere with the growth of susceptible rapidly proliferating malignant cells. The mechanism of action is thought to involve cross-linking of tumor cell DNA.
Cyclophosphamide is well absorbed after oral administration with a bioavailability greater than 75%. The unchanged drug has an elimination half-life of 3 to 12 hours. It is eliminated primarily in the form of metabolites, but from 5 to 25% of the dose is excreted in urine as unchanged drug. Several cytotoxic and noncytotoxic metabolites have been identified in urine and in plasma. Concentrations of metabolites reach a maximum in plasma 2 to 3 hours after an intravenous dose. Plasma protein binding of unchanged drug is low but some metabolites are bound to an extent greater than 60%. It has not been demonstrated that any single metabolite is responsible for either the therapeutic or toxic effects of cyclophosphamide. Although elevated levels of metabolites of cyclophosphamide have been observed in patients with renal failure, increased clinical toxicity in such patients has not been demonstrated.
Indications and Usage for Cytoxan
Malignant Diseases
CYTOXAN, although effective alone in susceptible malignancies, is more frequently used concurrently or sequentially with other antineoplastic drugs. The following malignancies are often susceptible to CYTOXAN treatment:
- Malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin’s disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma, histiocytic lymphoma, Burkitt’s lymphoma.
- Multiple myeloma.
- Leukemias: Chronic lymphocytic leukemia, chronic granulocytic leukemia (it is usually ineffective in acute blastic crisis), acute myelogenous and monocytic leukemia, acute lymphoblastic (stem-cell) leukemia in children (CYTOXAN given during remission is effective in prolonging its duration).
- Mycosis fungoides (advanced disease).
- Neuroblastoma (disseminated disease).
- Adenocarcinoma of the ovary.
- Retinoblastoma.
- Carcinoma of the breast.
Nonmalignant Disease Biopsy Proven “Minimal Change” Nephrotic Syndrome in Children
CYTOXAN is useful in carefully selected cases of biopsy proven “minimal change” nephrotic syndrome in children but should not be used as primary therapy. In children whose disease fails to respond adequately to appropriate adrenocorticosteroid therapy or in whom the adrenocorticosteroid therapy produces or threatens to produce intolerable side effects, CYTOXAN may induce a remission. CYTOXAN is not indicated for the nephrotic syndrome in adults or for any other renal disease.
Contraindications
Continued use of cyclophosphamide is contraindicated in patients with severely depressed bone marrow function. Cyclophosphamide is contraindicated in patients who have demonstrated a previous hypersensitivity to it. See WARNINGS and PRECAUTIONS.
Warnings
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Second malignancies have developed in some patients treated with cyclophosphamide used alone or in association with other antineoplastic drugs and/or modalities. Most frequently, they have been urinary bladder, myeloproliferative, or lymphoproliferative malignancies. Second malignancies most frequently were detected in patients treated for primary myeloproliferative or lymphoproliferative malignancies or nonmalignant disease in which immune processes are believed to be involved pathologically.
In some cases, the second malignancy developed several years after cyclophosphamide treatment had been discontinued. In a single breast cancer trial utilizing two to four times the standard dose of cyclophosphamide in conjunction with doxorubicin a small number of cases of secondary acute myeloid leukemia occurred within two years of treatment initiation. Urinary bladder malignancies generally have occurred in patients who previously had hemorrhagic cystitis. In patients treated with cyclophosphamide-containing regimens for a variety of solid tumors, isolated case reports of secondary malignancies have been published. One case of carcinoma of the renal pelvis was reported in a patient receiving long-term cyclophosphamide therapy for cerebral vasculitis. The possibility of cyclophosphamide-induced malignancy should be considered in any benefit-to-risk assessment for use of the drug.
Cyclophosphamide can cause fetal harm when administered to a pregnant woman and such abnormalities have been reported following cyclophosphamide therapy in pregnant women. Abnormalities were found in two infants and a six-month-old fetus born to women treated with cyclophosphamide. Ectrodactylia was found in two of the three cases. Normal infants have also been born to women treated with cyclophosphamide during pregnancy, including the first trimester. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Cyclophosphamide interferes with oogenesis and spermatogenesis. It may cause sterility in both sexes. Development of sterility appears to depend on the dose of cyclophosphamide, duration of therapy, and the state of gonadal function at the time of treatment. Cyclophosphamide-induced sterility may be irreversible in some patients.
Amenorrhea associated with decreased estrogen and increased gonadotropin secretion develops in a significant proportion of women treated with cyclophosphamide. Affected patients generally resume regular menses within a few months after cessation of therapy. Girls treated with cyclophosphamide during prepubescence generally develop secondary sexual characteristics normally and have regular menses. Ovarian fibrosis with apparently complete loss of germ cells after prolonged cyclophosphamide treatment in late prepubescence has been reported. Girls treated with cyclophosphamide during prepubescence subsequently have conceived.
Men treated with cyclophosphamide may develop oligospermia or azoospermia associated with increased gonadotropin but normal testosterone secretion. Sexual potency and libido are unimpaired in these patients. Boys treated with cyclophosphamide during prepubescence develop secondary sexual characteristics normally, but may have oligospermia or azoospermia and increased gonadotropin secretion. Some degree of testicular atrophy may occur. Cyclophosphamide-induced azoospermia is reversible in some patients, though the reversibility may not occur for several years after cessation of therapy. Men temporarily rendered sterile by cyclophosphamide have subsequently fathered normal children.
Urinary System
Hemorrhagic cystitis may develop in patients treated with cyclophosphamide. Rarely, this condition can be severe and even fatal. Fibrosis of the urinary bladder, sometimes extensive, also may develop with or without accompanying cystitis. Atypical urinary bladder epithelial cells may appear in the urine. These adverse effects appear to depend on the dose of cyclophosphamide and the duration of therapy. Such bladder injury is thought to be due to cyclophosphamide metabolites excreted in the urine. Forced fluid intake helps to assure an ample output of urine, necessitates frequent voiding, and reduces the time the drug remains in the bladder. This helps to prevent cystitis. Hematuria usually resolves in a few days after cyclophosphamide treatment is stopped, but it may persist. Medical and/or surgical supportive treatment may be required, rarely, to treat protracted cases of severe hemorrhagic cystitis. It is usually necessary to discontinue cyclophosphamide therapy in instances of severe hemorrhagic cystitis.
Cardiac Toxicity
Although a few instances of cardiac dysfunction have been reported following use of recommended doses of cyclophosphamide, no causal relationship has been established. Acute cardiac toxicity has been reported with doses as low as 2.4 g/m2 to as high as 26 g/m2, usually as a portion of an intensive antineoplastic multi-drug regimen or in conjunction with transplantation procedures. In a few instances with high doses of cyclophosphamide, severe, and sometimes fatal, congestive heart failure has occurred after the first cyclophosphamide dose. Histopathologic examination has primarily shown hemorrhagic myocarditis. Hemopericardium has occurred secondary to hemorrhagic myocarditis and myocardial necrosis. Pericarditis has been reported independent of any hemopericardium.
No residual cardiac abnormalities, as evidenced by electrocardiogram or echocardiogram appear to be present in patients surviving episodes of apparent cardiac toxicity associated with high doses of cyclophosphamide.
Cyclophosphamide has been reported to potentiate doxorubicin-induced cardiotoxicity.
Infections
Treatment with cyclophosphamide may cause significant suppression of immune responses. Serious, sometimes fatal, infections may develop in severely immunosuppressed patients. Cyclophosphamide treatment may not be indicated, or should be interrupted, or the dose reduced, in patients who have or who develop viral, bacterial, fungal, protozoan, or helminthic infections.
Precautions
General
Special attention to the possible development of toxicity should be exercised in patients being treated with cyclophosphamide if any of the following conditions are present.
- Leukopenia
- Thrombocytopenia
- Tumor cell infiltration of bone marrow
- Previous X-ray therapy
- Previous therapy with other cytotoxic agents
- Impaired hepatic function
- Impaired renal function
Laboratory Tests
During treatment, the patient’s hematologic profile (particularly neutrophils and platelets) should be monitored regularly to determine the degree of hematopoietic suppression. Urine should also be examined regularly for red cells which may precede hemorrhagic cystitis.
Drug Interactions
The rate of metabolism and the leukopenic activity of cyclophosphamide reportedly are increased by chronic administration of high doses of phenobarbital.
The physician should be alert for possible combined drug actions, desirable or undesirable, involving cyclophosphamide even though cyclophosphamide has been used successfully concurrently with other drugs, including other cytotoxic drugs.
Cyclophosphamide treatment, which causes a marked and persistent inhibition of cholinesterase activity, potentiates the effect of succinylcholine chloride.
If a patient has been treated with cyclophosphamide within 10 days of general anesthesia, the anesthesiologist should be alerted.
Adrenalectomy
Since cyclophosphamide has been reported to be more toxic in adrenalectomized dogs, adjustment of the doses of both replacement steroids and cyclophosphamide may be necessary for the adrenalectomized patient.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
See WARNINGS for information on carcinogenesis, mutagenesis, and impairment of fertility.
Nursing Mothers
Cyclophosphamide is excreted in breast milk. Because of the potential for serious adverse reactions and the potential for tumorigenicity shown for cyclophosphamide in humans, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety profile of CYTOXAN in pediatric patients is similar to that of the adult population (see ADVERSE REACTIONS).
Geriatric Use
Insufficient data from clinical studies of CYTOXAN for malignant lymphoma, multiple myeloma, leukemia, mycosis fungoides, neuroblastoma, retinoblastoma, and breast carcinoma are available for patients 65 years of age and older to determine whether they respond differently than younger patients. In two clinical trials in which cyclophosphamide was compared with paclitaxel, each in combination with cisplatin, for the treatment of advanced ovarian carcinoma, 154 (28%) of 552 patients who received cyclophosphamide plus cisplatin were 65 years or older. Subset analyses (<65 versus >65 years) from these trials, published reports of clinical trials of cyclophosphamide-containing regimens in breast cancer and non-Hodgkin’s lymphoma, and postmarketing experience suggest that elderly patients may be more susceptible to cyclophosphamide toxicities. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range and adjusting as necessary based on patient response (see DOSAGE AND ADMINISTRATION: Treatment of Malignant Diseases).
Adverse Reactions/Side Effects
Information on adverse reactions associated with the use of CYTOXAN (cyclophosphamide) is arranged according to body system affected or type of reaction. The adverse reactions are listed in order of decreasing incidence. The most serious adverse reactions are described in the WARNINGS section.
Digestive System
Nausea and vomiting commonly occur with cyclophosphamide therapy. Anorexia and, less frequently, abdominal discomfort or pain and diarrhea may occur. There are isolated reports of hemorrhagic colitis, oral mucosal ulceration and jaundice occurring during therapy. These adverse drug effects generally remit when cyclophosphamide treatment is stopped.
Skin and Its Structures
Alopecia occurs commonly in patients treated with cyclophosphamide. The hair can be expected to grow back after treatment with the drug or even during continued drug treatment, though it may be different in texture or color. Skin rash occurs occasionally in patients receiving the drug. Pigmentation of the skin and changes in nails can occur. Very rare reports of Stevens-Johnson syndrome and toxic epidermal necrolysis have been received during postmarketing surveillance; due to the nature of spontaneous adverse event reporting, a definitive causal relationship to cyclophosphamide has not been established.
Hematopoietic System
Leukopenia occurs in patients treated with cyclophosphamide, is related to the dose of drug, and can be used as a dosage guide. Leukopenia of less than 2000 cells/mm3 develops commonly in patients treated with an initial loading dose of the drug, and less frequently in patients maintained on smaller doses. The degree of neutropenia is particularly important because it correlates with a reduction in resistance to infections. Fever without documented infection has been reported in neutropenic patients.
Thrombocytopenia or anemia develop occasionally in patients treated with CYTOXAN. These hematologic effects usually can be reversed by reducing the drug dose or by interrupting treatment. Recovery from leukopenia usually begins in 7 to 10 days after cessation of therapy.
Urinary System
See WARNINGS for information on cystitis and urinary bladder fibrosis.
Hemorrhagic ureteritis and renal tubular necrosis have been reported to occur in patients treated with cyclophosphamide. Such lesions usually resolve following cessation of therapy.
Respiratory System
Interstitial pneumonitis has been reported as part of the postmarketing experience. Interstitial pulmonary fibrosis has been reported in patients receiving high doses of cyclophosphamide over a prolonged period.
Other
Anaphylactic reactions have been reported; death has also been reported in association with this event. Possible cross-sensitivity with other alkylating agents has been reported. SIADH (syndrome of inappropriate ADH secretion) has been reported with the use of cyclophosphamide. Malaise and asthenia have been reported as part of the postmarketing experience.
Overdosage
No specific antidote for cyclophosphamide is known. Overdosage should be managed with supportive measures, including appropriate treatment for any concurrent infection, myelosuppression, or cardiac toxicity should it occur.
Cytoxan Dosage and Administration
Treatment of Malignant Diseases Adults and Children
When used as the only oncolytic drug therapy, the initial course of CYTOXAN for patients with no hematologic deficiency usually consists of 40 to 50 mg/kg given intravenously in divided doses over a period of 2 to 5 days. Other intravenous regimens include 10 to 15 mg/kg given every 7 to 10 days or 3 to 5 mg/kg twice weekly.
Oral CYTOXAN dosing is usually in the range of 1 to 5 mg/kg/day for both initial and maintenance dosing.
Many other regimens of intravenous and oral CYTOXAN have been reported. Dosages must be adjusted in accord with evidence of antitumor activity and/or leukopenia. The total leukocyte count is a good, objective guide for regulating dosage. Transient decreases in the total white blood cell count to 2000 cells/mm3 (following short courses) or more persistent reduction to 3000 cells/mm3 (with continuing therapy) are tolerated without serious risk of infection if there is no marked granulocytopenia.
When CYTOXAN is included in combined cytotoxic regimens, it may be necessary to reduce the dose of CYTOXAN as well as that of the other drugs.
CYTOXAN and its metabolites are dialyzable although there are probably quantitative differences depending upon the dialysis system being used. Patients with compromised renal function may show some measurable changes in pharmacokinetic parameters of CYTOXAN metabolism, but there is no consistent evidence indicating a need for CYTOXAN dosage modification in patients with renal function impairment.
Treatment of Nonmalignant Diseases Biopsy Proven “Minimal Change’’ Nephrotic Syndrome in Children
An oral dose of 2.5 to 3 mg/kg daily for a period of 60 to 90 days is recommended. In males, the incidence of oligospermia and azoospermia increases if the duration of CYTOXAN treatment exceeds 60 days. Treatment beyond 90 days increases the probability of sterility. Adrenocorticosteroid therapy may be tapered and discontinued during the course of CYTOXAN therapy. See PRECAUTIONS concerning hematologic monitoring.
Preparation and Handling of Solutions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Add the diluent to the vial and shake it vigorously to dissolve. If the powder fails to dissolve immediately and completely, it is advisable to allow the vial to stand for a few minutes. Use the quantity of diluent shown below to constitute the product:
| Dosage Strength | CYTOXAN Contains Cyclophosphamide Monohydrate | Quantity of Diluent |
| 500 mg | 534.5 mg | 25 mL |
| 1 g | 1069.0 mg | 50 mL |
| 2 g | 2138.0 mg | 100 mL |
For Direct Injection
CYTOXAN should be prepared for parenteral use by adding 0.9% sterile sodium chloride solution. Solutions of CYTOXAN may be injected intravenously, intramuscularly, intraperitoneally, or intrapleurally if constituted by adding 0.9% sterile sodium chloride solution.
For Infusion
CYTOXAN may be prepared for parenteral use by infusion using any of the following methods:
- CYTOXAN constituted with 0.9% sterile sodium chloride may be infused without further dilution.
- CYTOXAN constituted with 0.9% sterile sodium chloride may be infused following further dilution in the following:
Dextrose Injection, USP (5% dextrose)
Dextrose and Sodium Chloride Injection, USP (5% dextrose and 0.9% sterile sodium chloride)
5% Dextrose and Ringer’s Injection
Lactated Ringer’s Injection, USP
Sodium Chloride Injection, USP (0.45% sterile sodium chloride)
Sodium Lactate Injection, USP (1/6 molar sodium lactate)
- CYTOXAN sterile powder may be prepared for parenteral use by infusion by adding Sterile Water for Injection, USP. CYTOXAN, constituted in water, is hypotonic and should not be injected directly. Prior to infusion, solutions of sterile powder constituted in Sterile Water for Injection, USP must be further diluted in one of the following:
Dextrose Injection, USP (5% dextrose)
Dextrose and Sodium Chloride Injection, USP (5% dextrose and 0.9% sterile sodium chloride)
5% Dextrose and Ringer’s Injection
Lactated Ringer’s Injection, USP
Sodium Chloride Injection, USP (0.45% sterile sodium chloride)
Sodium Lactate Injection, USP (1/6 molar sodium lactate)
Stability of Constituted Parenteral Solutions
CYTOXAN (prepared for either direct injection or infusion) is chemically and physically stable for 24 hours at room temperature or for six days in the refrigerator; it does not contain any antimicrobial preservative and thus care must be taken to assure the sterility of prepared solutions.
The osmolarities of solutions of CYTOXAN constituted with water and 0.9% sterile sodium chloride solution are found in the following table:
| CYTOXAN and Diluent | mOsm/L |
| 5 mL water per 100 mg cyclophosphamide (anhydrous) | 74 |
| 5 mL 0.9% sterile sodium chloride solution per 100 mg cyclophosphamide (anhydrous) | 374 |
Isotonic 0.9% sterile sodium chloride solution has an osmolarity of 289 mOsm/L.
How is Cytoxan supplied
CYTOXAN® contains cyclophosphamide monohydrate and is supplied in vials for single dose use.
CYTOXAN (cyclophosphamide for injection, USP).
| NDC 0015-0502-41 | 500 mg vial, carton of 1 |
| NDC 0015-0505-41 | 1.0 g vial, carton of 1 |
| NDC 0015-0506-41 | 2.0 g vial, carton of 1 |
Store vials at or below 77° F (25° C). During transport or storage of CYTOXAN vials, temperature influences can lead to melting of the active ingredient, cyclophosphamide. Vials containing melted substance can be visually differentiated. Melted cyclophosphamide is a clear or yellowish viscous liquid usually found as a connected phase or in droplets in the affected vials. Do not use CYTOXAN vials if there are signs of melting.
CYTOXAN® Tablets, 25 mg and CYTOXAN Tablets, 50 mg, are white tablets with blue flecks containing 25 mg and 50 mg cyclophosphamide (anhydrous), respectively.
CYTOXAN Tablets (cyclophosphamide tablets, USP).
| NDC 0015-0503-01 | 50 mg, bottles of 100 |
| NDC 0015-0504-01 | 25 mg, bottles of 100 |
Store tablets at or below 77° F (25° C); tablets will withstand brief exposure to temperatures up to 86° F (30° C) but should be protected from temperatures above 86° F (30° C).
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
To minimize the risk of dermal exposure, always wear impervious gloves when handling vials containing CYTOXAN sterile powder for injection, or bottles containing CYTOXAN tablets. This includes all handling activities in clinical settings, pharmacies, storerooms, and home healthcare settings, including during unpacking and inspection, transport within a facility, and dose preparation and administration.
References
- ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 1999:32-41.
- Recommendations for the safe handling of parenteral antineoplastic drugs. Washington, DC: Division of Safety, National Institutes of Health; 1983. US Dept of Health and Human Services, Public Health Service publication NIH 83-2621.
- AMA Council on Scientific Affairs. Guidelines for handling parenteral antineoplastics. JAMA. 1985;253:1590-1592.
- National Study Commission on Cytotoxic Exposure. Recommendations for handling cytotoxic agents. 1987. Available from Louis P. Jeffrey, Chairman, National Study Commission on Cytotoxic Exposure. Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, MA 02115.
- Clinical Oncological Society of Australia. Guidelines and recommendations for safe handling of antineoplastic agents. Med J Aust. 1983;1:426-428.
- Jones RB, Frank R, Mass T. Safe handling of chemotherapeutic agents: a report from The Mount Sinai Medical Center. CA Cancer J Clin. 1983;33:258-263.
- American Society of Hospital Pharmacists. ASHP technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990;47:1033-1049.
- Controlling occupational exposure to hazardous drugs. (OSHA Work-Practice Guidelines.) Am J Health-Syst Pharm. 1996;53:1669-1685.
Vials Manufactured by:
Baxter Healthcare Corporation
Deerfield, Illinois 60015 USA
Vials Made in Germany
Vials Distributed by:
Bristol-Myers Squibb Company
Princeton, New Jersey 08543
Tablets Manufactured by:
Bristol-Myers Squibb Company
Princeton, New Jersey 08543
Tablets Made in Italy
1175025A2
1050971A4
USA 5638 0612 C 669
Rev September 2005
---------------------------------------------
REPRESENTATIVE PACKAGING
See How Supplied section for a complete list of available packages of CYTOXAN.
NDC 0015-0502-41
CYTOXAN®
(cyclophosphamide for injection, USP)
500 mg
WARNING: CYTOTOXIC AGENT
Store vial at or below 77° F (25° C).
Rx only

NDC 0015-0505-41
CYTOXAN®
(cyclophosphamide for injection, USP)
1 gram
WARNING: CYTOTOXIC AGENT
Store vial at or below 77° F (25° C).
Rx only

| CYTOXAN
cyclophosphamide injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYTOXAN
cyclophosphamide injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYTOXAN
cyclophosphamide injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYTOXAN
cyclophosphamide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYTOXAN
cyclophosphamide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - E.R. Squibb & Sons, L.L.C. (006370092) |
Frequently asked questions
More about Cytoxan (cyclophosphamide)
- Check interactions
- Compare alternatives
- Reviews (7)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: alkylating agents
- Breastfeeding



