Raplixa Prescribing Information
Package insert / product label
Generic name: fibrinogen human and thrombin human
Dosage form: powder
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
RAPLIXA® (Fibrin Sealant (Human))
Powder for topical use only
Initial U.S. Approval: 2015
Indications and Usage for Raplixa
RAPLIXA is a fibrin sealant indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical.
RAPLIXA is used in conjunction with an absorbable gelatin sponge (USP) and is applied directly or using the RaplixaSpray™ device. (1)
Raplixa Dosage and Administration
For topical use only.
Do not reconstitute. Use within one hour after opening.
The required dose of RAPLIXA depends on the size of the bleeding area. The maximum total dose of RAPLIXA per surgery is 3 grams. (2)
Applying to the surface of bleeding tissue only, administer RAPLIXA directly from the vial or using the RaplixaSpray delivery device. RAPLIXA may be used at multiple bleeding sites in the same patient. Use no more than two vials of RAPLIXA with the RaplixaSpray device. To administer a third vial, open a new device. (2)
Dosage Forms and Strengths
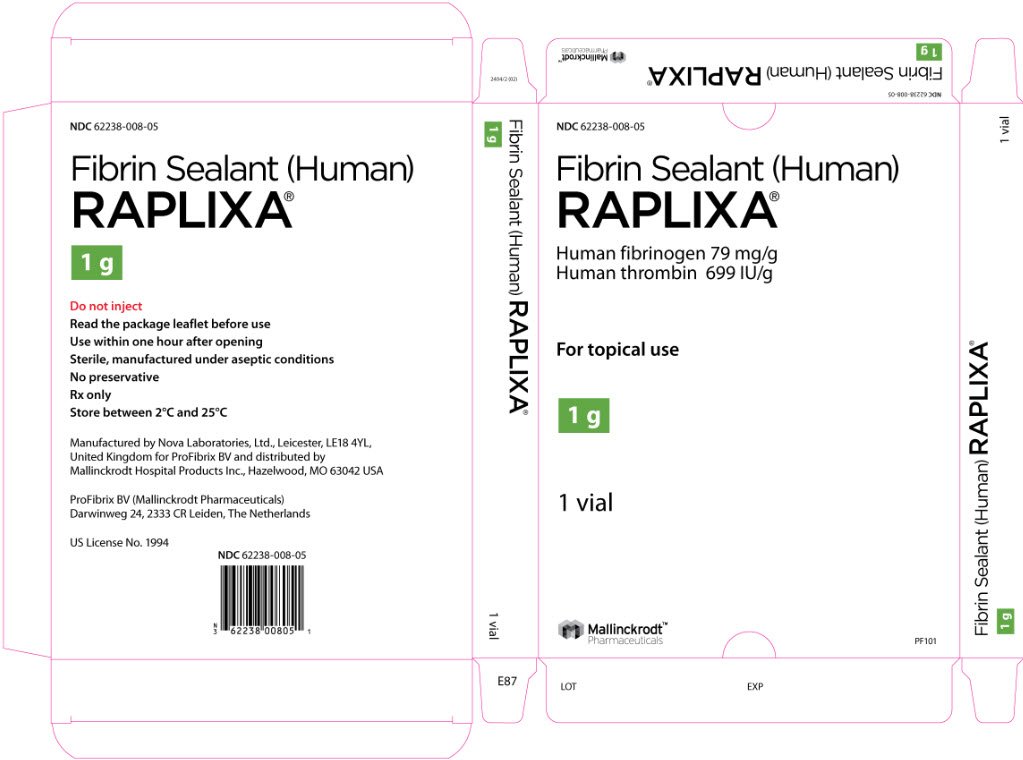
RAPLIXA is available as dry, ready-to-use powder containing nominally 79 mg human fibrinogen and 699 international units human thrombin per gram of powder. RAPLIXA is supplied in single use glass vials in three presentations: 0.5 gram, 1 gram, and 2 grams per vial. (3)
Contraindications
Do not use:
- Intravascularly.
- For the treatment of severe or brisk arterial bleeding.
- In patients known to have anaphylactic or severe systemic reactions to human blood products. (4)
Warnings and Precautions
- Thromboembolic events may result from intravascular application of RAPLIXA. (5.1)
- Air or gas embolism can occur using air- or gas-pressurized sprayers to administer fibrin sealants. Operate the device according to the manufacturer’s instructions. (5.2)
- RAPLIXA may carry a risk of transmitting infectious agents, such as viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agents, despite manufacturing steps designed to reduce the risk of viral transmission. (5.3)
- Allergic type hypersensitivity reactions may occur.If allergic type hypersensitivity symptoms occur, discontinue administration immediately. (5.4)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (> 5% subjects) were procedural pain, nausea, constipation, pyrexia, and hypotension. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mallinckrodt Hospital Products Inc. at 1-800-778-7898 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pregnancy: No human or animal data. Use only if clearly needed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2017
Full Prescribing Information
1. Indications and Usage for Raplixa
RAPLIXA is a fibrin sealant indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical.
RAPLIXA is used in conjunction with an absorbable gelatin sponge (USP) and may be applied directly or using the RaplixaSpray device.
2. Raplixa Dosage and Administration
For topical use only.
Do not reconstitute. Use within one hour of opening.
2.1 Dose
The required amount of RAPLIXA needed to stop bleeding varies and is based on the size of the bleeding area to be treated. The maximum total dose of RAPLIXA per surgery is 3 grams. In clinical trials, it was demonstrated that smaller bleeding sites covering an area of less than 10 cm2 used 0.5 gram to 1.0 gram of RAPLIXA. Larger bleeding sites covering an area of 10-100 cm2 used 1.0 to 2.0 grams of RAPLIXA to stop bleeding. Using the RaplixaSpray device, 1.0 gram can cover a 100 cm2 bleeding surface area.
The required dose of RAPLIXA depends on the size of the bleeding area to be treated according to Table 1 below.
| Maximum Surface Area Direct Application from Vial | Maximum Surface Area Application Using RaplixaSpray Device | RAPLIXA Package Size |
| 25 cm2 | 50 cm2 | 0.5 g |
| 50 cm2 | 100 cm2 | 1 g |
| 100 cm2 | 200 cm2 | 2 g |
2.2 Administration
Applying to the surface of bleeding tissue only, administer RAPLIXA directly from the vial or using the RaplixaSpray device. RAPLIXA may be used at multiple bleeding sites in the same patient. Use no more than two vials of RAPLIXA with the RaplixaSpray device. To administer a third vial, open a new device.
Direct Application of RAPLIXA with Absorbable Gelatin Sponge (USP)
Note: Refer to Absorbable Gelatin Sponge (USP) labeling for complete instructions for use.
- Open the pouch and take out the RAPLIXA vial following standard sterile technique to ensure that the vial and surgical field remain sterile.
- Check that the powder is in the bottom of the vial. Remove the flip-off top, leaving the stopper in place until immediately prior to use.
- Prepare sterile gelatin sponge by trimming to an appropriate size for the bleeding site.
- Remove stopper and sprinkle a uniform thin coating of RAPLIXA gently onto bleeding site and apply gentle pressure with gelatin sponge using sterile gauze.
OR
- Remove stopper and sprinkle a thin layer of RAPLIXA gently onto a pre-wetted gelatin sponge and place onto the bleeding site with gentle pressure using sterile gauze.
Preparation and Application of RAPLIXA Using the RaplixaSpray Device with Absorbable Gelatin Sponge (USP)
Note: Refer to the RaplixaSpray Device labeling for complete instructions for use. Refer to Absorbable Gelatin Sponge (USP) labeling for complete instructions for use.
- Prepare the pressure regulator (Air or CO2) according to the manufacturer’s instructions for use.
- Open the pouch and take out the RAPLIXA vial following standard sterile technique to ensure that the vial and surgical field remain sterile.
- Check that the powder is in the bottom of the vial. Remove the flip-off top, leaving the stopper in place until use.
- Prepare gelatin sponge by trimming to an appropriate size for the bleeding site.
- To attach the vial to the RaplixaSpray device, invert the device and place the upright vial into the gray rubber ring on the device, turn the device upright and return the device to the sterile field until use.
- Activate air or gas flow.
- Device is now ready for use. DO NOT push button until ready for use.
- Check that the pressure is 1.5 bar (22 psi).
- Ensure the vial is kept within 45° of vertical at all times.
- Hold nozzle at a minimum distance of 5 cm (or 2 inches) from the bleeding site.
- Start application by gently pressing the operating button.
- Powder should cover the bleeding surface as a uniform thin coating. Apply RAPLIXA within 10-60 seconds.
- Immediately after RAPLIXA application, place a gelatin sponge, trimmed to the approximate size, on top of the RAPLIXA powder. The gelatin sponge may be used dry or moistened with sterile saline. A moistened sponge molds more easily to irregularly-shaped and contoured bleeding areas. Hold the gelatin sponge in place with manual pressure using sterile gauze.
- Hold the device upside down and carefully remove the empty vial. If needed, attach the second vial (repeat steps 1-14). Use no more than two vials of RAPLIXA with the RaplixaSpray device.
- To administer a third vial, open a new RaplixaSpray delivery device (repeat steps 1-13).
3. Dosage Forms and Strengths
RAPLIXA is available as dry, ready-to-use powder containing 79 mg human fibrinogen and 699 international units human thrombin per gram of powder. RAPLIXA is supplied in single-use glass vials in three sizes: 0.5 gram, 1 gram, and 2 grams per vial.
4. Contraindications
- Do not apply intravascularly.
- Do not use for the treatment of severe or brisk arterial bleeding.
- Do not use in patients known to have anaphylactic or severe systemic reactions to human blood products.
5. Warnings and Precautions
5.1 Thrombosis
Life-threatening thromboembolic events may result from intravascular application of RAPLIXA.
5.2 Air or Gas Embolism
Air or gas embolism has occurred with the use of spray devices employing pressure regulators to administer fibrin sealants. This appears to be related to the use of the spray device at higher than manufacturer recommended pressures and in close proximity to the tissue surface. Reports with the use of other devices describe air emboli that are life-threatening and include fatality. To minimize this risk, operate the RaplixaSpray device according to the manufacturer’s instructions. In the absence of instructions, operate at a maximum air pressure of 1.5 bar (22 psi) and at a minimum distance of 5 cm (2 inches) from the bleeding surface. Monitor changes in blood pressure, pulse, oxygen saturation, and end tidal CO2 for signs and symptoms of embolism.
5.3 Transmissible Infectious Agents
Because the biological components of this product are made of human plasma, RAPLIXA may carry a risk of transmitting infectious agents, such as viruses the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite manufacturing steps designed to reduce the risk of infectious agent transmission. The risk of transmitting an infectious agent has been minimized by screening plasma donors for prior exposure to certain infectious agents, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products.
All infections considered by a physician to possibly have been transmitted by this product should be reported by the physician or other healthcare provider to Mallinckrodt Hospital Products Inc. at 1-800-778-7898.
6. Adverse Reactions/Side Effects
The most commonly reported adverse reactions (> 5% subjects) were procedural pain, nausea, constipation, pyrexia, and hypotension.
6.1 Clinical Trials Experience
The RAPLIXA clinical trials safety data base consists of two randomized, single-blind, controlled Phase 2 trials and one randomized single-blind controlled Phase 3 trial. All of the trials evaluated the safety and immunogenicity of RAPLIXA topically applied with a gelatin sponge and included patients undergoing spinal surgery, vascular surgery, hepatic resection, soft tissue dissection/general surgery. These trials resulted in an overall safety database of 566 patients treated with RAPLIXA with gelatin sponge. Most patients (94%) were exposed to a 1 gram vial of RAPLIXA. Overall, the incidence of adverse reactions was similar between treatment groups (Table 2).
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
|
a FC-002 US and FC-002 NL clinical trials combined |
||||||
| Phase 2 a | Phase 3 b | Total c | ||||
| N (%) of Patients Preferred Term | R+G d
(N=86) | G d
(N=39) | R+G d
(N=480) | G d
(N=239) | R+G d
(N=566) | G d
(N=278) |
| Procedural pain | 40 (47) | 16 (41) | 257 (54) | 134 (56) | 297 (52) | 150 (54) |
| Nausea | 26 (30) | 13 (33) | 120 (25) | 48 (20) | 146 (26) | 61 (22) |
| Constipation | 21 (24) | 9 (23) | 72 (15) | 31 (13) | 93 (16) | 40 (14) |
| Incision site pain | 5 (6) | 3 (8) | 63 (13) | 32 (13) | 68 (12) | 35 (13) |
| Pyrexia | 7 (8) | 5 (13) | 37 (8) | 11 (5) | 44 (8) | 16 (6) |
| Anemia | 4 (5) | 2 (5) | 33 (7) | 17 (7) | 37 (7) | 19 (7) |
| Vomiting | 11 (13) | 2 (5) | 26 (5) | 12 (5) | 37 (7) | 14 (5) |
| Hypotension | 2 (2) | 2 (5) | 38 (8) | 16 (7) | 40 (7) | 18 (6) |
| Pruritus | 3 (3) | 1 (3) | 33 (7) | 8 (3) | 36 (6) | 9 (3) |
| Hypertension | 1 (1) | 0 | 25 (5) | 10 (4) | 26 (5) | 10 (4) |
Immunogenicity
The incidence of antibody formation in the Phase 3 trial was evaluated. Antibodies were detectable at baseline in 9/440 of RAPLIXA-treated patients (2%) and 9/222 of gelatin sponge-treated patients (4%). Nine of 440 patients (2%) in the RAPLIXA group and 6 of 222 patients (3%) in the gelatin sponge alone group developed anti-thrombin antibodies (non-neutralizing) during the trial. The clinical significance of these antibodies is not known.
Anti-fibrinogen antibodies were not detected pre- or post-treatment in any subjects that were positive for anti-thrombin antibodies.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to RAPLIXA with the incidence of antibodies to other products may be misleading.
8. Use In Specific Populations
8.1 Pregnancy
Animal reproduction studies have not been conducted with RAPLIXA. It is also not known whether RAPLIXA can cause fetal harm when administered to pregnant women or can affect reproductive capacity. RAPLIXA should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether RAPLIXA is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when administering to a nursing woman.
11. Raplixa Description
RAPLIXA is a fibrin sealant comprised of human plasma-derived fibrinogen and thrombin that is designed to be used as an adjunct to surgical hemostasis. Each component is separately spray dried with trehalose followed by blending the two components to provide a ready-to-use, pre-mixed, sterile, dry powder that is filled in sterile medical grade glass vials. RAPLIXA is manufactured aseptically, resulting in a sterile product in a sterile vial. RAPLIXA does not contain any preservatives.
Except for fibrinogen and thrombin, the product contains the following components added during the manufacturing: trehalose – 824 mg/g, calcium chloride – 11 mg/g, and traces of the components from the formulations of fibrinogen and thrombin raw materials: human albumin, sodium chloride, sodium citrate, and L-Arginine hydrochloride.
Viral Clearance
All human plasma used in the manufacture of RAPLIXA is tested for the presence of current specific virus infections using FDA-licensed serological assays and nucleic acid testing (NAT) assays for HBV, HIV-1/2, and HCV and found to be non-reactive (negative). The manufacturing procedures for fibrinogen and thrombin include processing steps designed to reduce the risk of viral transmission, including pasteurization, precipitation and adsorption steps.
Validation studies for fibrinogen and thrombin manufacturing steps were conducted for their capacity to inactivate and/or remove viruses. These in vitro validation studies were conducted, using samples from manufacturing intermediates spiked with virus suspensions of known titers followed by further processing under conditions equivalent to those in the respective manufacturing steps. The cumulative virus reduction factors (expressed as log10) are shown in Table 3 for each virus tested.
|
a HIV-1: Human Immunodeficiency Virus 1, HSV: Herpes Simplex Virus, BVDV: Bovine Viral Diarrhea Virus, CPV: Canine Parvo Virus: a model for B19V, HAV: Hepatitis A Virus, PRV: Pseudorabies Virus |
|||||
| Cumulative Reduction Factors for Virus Removal/Inactivation of Human Thrombin | |||||
| Reduction Factors [log10] of Virus a tested | |||||
| Manufacturing step | HIV-1 | HSV | BVDV | CPV | HAV |
| Pasteurization, precipitation and adsorption steps | ≥19.6 | ≥21.4 | ≥13.4 | 6.6 | 8.7 |
| Cumulative Reduction Factors for Virus Removal/Inactivation of Human Fibrinogen | |||||
| Reduction Factors [log10] of Virus a tested | |||||
| Manufacturing step | HIV-1 | HSV b | BVDV | CPV | HAV |
| Pasteurization, precipitation and adsorption steps | ≥9.6 | ≥9.1 | ≥11.2 | ≥4.4 | ≥6.7 |
12. Raplixa - Clinical Pharmacology
12.1 Mechanism of Action
RAPLIXA contains a spray-dried mixture of human plasma-derived fibrinogen and human plasma-derived thrombin powders that are designed to mimic the final steps in the coagulation cascade. RAPLIXA dissolves readily on contact with aqueous fluids (e.g., blood) activating thrombin which triggers an immediate conversion of fibrinogen into fibrin, and subsequent clot formation.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of RAPLIXA or studies to determine the effects of RAPLIXA on genotoxicity or fertility have not been performed. An assessment of the carcinogenic potential of RAPLIXA was completed and suggests minimal carcinogenic risk from product use.
13.2 Animal Toxicology and/or Pharmacology
Single or multiple dose implantation studies applying RAPLIXA with the absorbable gelatin sponge (USP) into liver or spleen surgical wounds showed progressive biodegradation of RAPLIXA, consistent with metabolism via fibrinolysis and phagocytosis. Approximately 5 to 10% of RAPLIXA and the carrier gelatin sponge remained at the application site at study termination 12 weeks after surgery.
14. Clinical Studies
RAPLIXA (plus gelatin sponge) was evaluated in a randomized (2:1), single-blind, controlled clinical study against absorbable gelatin sponge (USP) in mild (oozing and/or capillary leakage) to moderate (gradual and steady flow) surgical bleeding. RAPLIXA was applied directly or using the RaplixaSpray device. The study enrolled approximately 180 subjects in each of four surgical indications: spinal surgery, hepatic resection, vascular surgery, and soft tissue dissection. Each surgical indication was evaluated separately for efficacy.
The objective of the study was to demonstrate the superiority of RAPLIXA (applied directly or using RaplixaSpray) plus gelatin sponge, as compared to gelatin sponge alone. Efficacy was evaluated by time to hemostasis within the 5 minute observation period.
The study included 721 subjects randomized in a single-blinded manner during surgery, in a 2:1 ratio to treatment with RAPLIXA plus gelatin sponge (active group) or gelatin sponge alone (control group) after an appropriate target bleeding site (TBS) was identified. There were 181 subjects treated for soft tissue surgery, 183 subjects treated for spinal surgery, 180 subjects treated for hepatic resection and 175 subjects treated for vascular surgery.
Results of this study demonstrated that treatment with RAPLIXA plus gelatin sponge was superior in achieving hemostasis compared to treatment with gelatin sponge alone. These results were statistically significant in the efficacy (n=719) and ITT (n=721) populations. Table 4 summarizes the primary efficacy endpoint of the time to hemostasis within 5 minutes in the efficacy population.
| a RAPLIXA + Gelatin Sponge n=122; Gelatin Sponge Alone n=61 b RAPLIXA + Gelatin Sponge n=117; Gelatin Sponge Alone n=58 c RAPLIXA + Gelatin Sponge n=119; Gelatin Sponge Alone n=61 d RAPLIXA + Gelatin Sponge n=122; Gelatin Sponge Alone n=59 e Log Rank Test |
||||
| Surgery Type | ||||
| Spinal (n=183) a | Vascular (n=175) b | Hepatic Resection (n=180) c | Soft Tissue Dissection (n=181) d |
|
| Median TTH: RAPLIXA plus gelatin sponge (95% CI) | 1.0 (-, -) | 2.0 (1.5, 2.5) | 1.0 (1.0, 1.5) | 1.5 (1.0, 1.5) |
| Median TTH: gelatin sponge (95% CI) | 2.5 (2, 3) | 4.0 (3, 5) | 2.0 (1.5, 2.5) | 2.5 (2.0, 3.5) |
| P value e | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Cox proportional hazard ratio (95% CI) | 3.3 [2.3 , 4.7] | 2.1 [1.5 , 3.1] | 2.3 [1.6 , 3.2] | 3.4 [2.3 , 4.8] |
15. References
Bochicchio GV, Gupta N, Porte RJ, et al. The FINISH-3 trial: a phase 3, international, randomized, single-blind, controlled trial of topical Fibrocaps in intraoperative surgical hemostasis. J Am Coll Surg. 2015: 220(1): 70-81.
Verhoef C, Singla N, Moneta G, et al. Fibrocaps for surgical hemostasis: two randomized, controlled phase II trials. J Surg Res. 2015:194(2):679-87.
16. How is Raplixa supplied
How Supplied
The RAPLIXA powder is supplied in sterile medical grade single-use glass vials with rubber stopper and aluminum tear-off crimp seal with a white polypropylene flip-off, packaged in an aluminum pouch, and contains no preservative.
RAPLIXA is supplied in three different presentations: 0.5 gram (NDC 62238-008-01), 1 gram (NDC 62238-008-02), and 2 gram (NDC 62238-008-03) vials.
Storage and Handling
- Store RAPLIXA vials at 2°C to 25°C (36°F to 77°F). Refrigeration is not required.
- Do not freeze.
- Do not use beyond the expiration date printed on the carton or vial.
- Use RAPLIXA powder within one hour after opening a vial.
17. Patient Counseling Information
- Advise patients to consult their physician if they experience chest pain, shortness of breath, difficulty speaking or swallowing, leg tenderness or swelling, or other symptoms of thromboembolism.
- Inform patients that RAPLIXA may carry a risk of transmitting infectious agents, (e.g., viruses such as hepatitis A and parvovirus B19 and theoretically the CJD agent). Instruct patients to consult their physician if symptoms of B19 virus infection (fever, drowsiness, and chills, followed two weeks later by a rash and joint pain) or hepatitis A (several days to weeks of poor appetite, fatigue and low-grade fever followed by nausea, vomiting and abdominal pain, dark urine, yellowed complexion) appear.
Mallinckrodt, the “M” brand mark, the Mallinckrodt Pharmaceuticals logo and other brands are trademarks of a Mallinckrodt company.
© 2016 Mallinckrodt.
Manufactured by Nova Laboratories, Ltd., Leicester, LE18 4YL, United Kingdom for ProFibrix BV and distributed by Mallinckrodt Hospital Products Inc., Hazelwood, MO 63042 USA
ProFibrix BV (Mallinckrodt Pharmaceuticals), Darwinweg 24, 2333 CR Leiden, The Netherlands
US License No. 1994
Mallinckrodt™
Pharmaceuticals
| RAPLIXA
fibrinogen human and thrombin human powder |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ProFibrix BV (408491293) |