Duzallo Prescribing Information
Package insert / product label
Generic name: lesinurad and allopurinol
Dosage form: tablet, film coated
Drug class: Antihyperuricemic agents
Medically reviewed by Drugs.com. Last updated on Jan 18, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
DUZALLO® (lesinurad and allopurinol) tablets, for oral use
Initial U.S. Approval: 2017
Indications and Usage for Duzallo
DUZALLO, a combination of lesinurad, a URAT1 inhibitor, and allopurinol, a xanthine oxidase inhibitor, is indicated for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a medically appropriate daily dose of allopurinol alone. (1)
Limitations of Use:
- DUZALLO is not recommended for the treatment of asymptomatic hyperuricemia. (1.1)
Duzallo Dosage and Administration
- The recommended dose of DUZALLO is one 200 mg lesinurad/300 mg allopurinol tablet per day (or one 200 mg lesinurad/200 mg allopurinol tablet per day for patients with renal impairment (45 - < 60 mL/min eCLcr) on a medically appropriate dose of 200 mg allopurinol (2.2)).
- Use one tablet of DUZALLO in place of an equivalent portion of the total daily allopurinol dose. The total daily dose of allopurinol should be maintained at the time of initiating DUZALLO. (2.1)
- One tablet of DUZALLO contains the maximum daily lesinurad dose (200 mg). (2.1)
- Do not take more than 1 tablet of DUZALLO per day. (2.1)
- Do not combine DUZALLO with ZURAMPIC® (lesinurad). (2.1)
- Use of DUZALLO is not recommended for patients taking daily doses of allopurinol less than 300 mg (or less than 200 mg in patients with moderate renal impairment). (2.1)
- DUZALLO tablets should be taken in the morning with food and water. (2.1)
- Patients should be instructed to stay well hydrated. (2.1)
- Assess renal function before initiating DUZALLO. Do not initiate DUZALLO if eCLcr is below 45 mL/min. (2.2)
- Discontinue DUZALLO if eCLcr persistently falls below 45 mL/min. (2.2)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Renal events: Adverse reactions related to renal function, including acute renal failure, have occurred after initiating lesinurad, one of the components of DUZALLO. A higher incidence has occurred at the 400 mg dose of lesinurad than at the 200 mg dose. Monitor renal function at initiation and during therapy with DUZALLO, particularly in patients with eCLcr below 60 mL/min, and evaluate for signs and symptoms of acute uric acid nephropathy. (5.1)
- Skin Rash and Hypersensitivity: DUZALLO, should be discontinued at the first appearance of skin rash or other signs that may indicate an allergic reaction, as allopurinol has been associated with severe hypersensitivity (some resulting in death). (5.2)
- Hepatotoxicity: Hepatotoxicity has been reported in patients on allopurinol. Inform patients of warning signs and symptoms of hepatotoxicity. If symptoms develop, liver function evaluation should be performed. (5.3)
- Cardiovascular events: Major adverse cardiovascular events were observed with lesinurad; a causal relationship has not been established. (5.4)
- Bone Marrow Suppression: Bone marrow depression affecting one or more cell lines has been reported with allopurinol. (5.5)
Adverse Reactions/Side Effects
- Most common adverse reactions in 12-month controlled clinical trials (occurring in greater than or equal to 2% of patients treated with lesinurad in combination with a xanthine oxidase inhibitor and more frequently than on xanthine oxidase inhibitor alone) were headache, influenza, blood creatinine increased, and gastroesophageal reflux disease. (6.1)
- The most frequently reported adverse reaction for allopurinol is skin rash. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Ironwood Pharmaceuticals, Inc. at 1-844-374-4793 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Mercaptopurine or Azathioprine: Reduce mercaptopurine or azathioprine dose to approximately one-third to one-fourth of the usual dose and closely monitor for therapeutic response and the appearance of toxicity. (5.5, 7.2)
- Coumarin Anticoagulants: Carefully monitor prothrombin time. (5.6, 7.2)
- Moderate Cytochrome P450 2C9 (CYP2C9) Inhibitors: Use DUZALLO with caution. (7.1)
- CYP3A Substrates: Monitor for efficacy of the CYP3A substrate. (7.1)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2017
Related/similar drugs
allopurinol, febuxostat, Zyloprim, probenecid, Uloric, Krystexxa
Full Prescribing Information
WARNING: RISK OF ACUTE RENAL FAILURE
- Acute renal failure has occurred with lesinurad, one of the components of DUZALLO. [see Warning and Precautions (5.1), Adverse Reactions (6.1)]
1. Indications and Usage for Duzallo
DUZALLO®, a combination of lesinurad, a uric acid transporter 1 (URAT1) inhibitor, and allopurinol, a xanthine oxidase inhibitor, is indicated for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with a medically appropriate daily dose of allopurinol alone [see Clinical Studies (14)].
2. Duzallo Dosage and Administration
2.1 Recommended Dosing
DUZALLO tablets are for oral use. DUZALLO should be taken once daily by mouth, in the morning with food and water. Patients should be instructed to stay well hydrated (e.g., 2 liters of liquid per day).
One tablet of DUZALLO contains the maximum daily lesinurad dose (200 mg). Do not take more than 1 tablet of DUZALLO per day. Do not combine DUZALLO with ZURAMPIC® (lesinurad).
Use of DUZALLO is not recommended for patients taking daily doses of allopurinol less than 300 mg (or less than 200 mg in patients with estimated creatinine clearance [eCLcr] less than 60 mL/min).
Use one tablet of DUZALLO in place of an equivalent portion of the total daily allopurinol dose. The total daily dose of allopurinol should be maintained at the time of initiating DUZALLO.
- For patients who have not achieved target serum uric acid on a medically appropriate dose of allopurinol > 300 mg, DUZALLO may be initiated by using one tablet of DUZALLO in place of an equivalent portion of the total daily allopurinol dose.
- For patients who have not achieved target serum uric acid on a medically appropriate dose of allopurinol of 300 mg, DUZALLO may be initiated by using one tablet of DUZALLO 200 mg lesinurad /300 mg allopurinol daily in place of 300 mg of allopurinol.
- For patients who have not achieved target serum uric acid on a medically appropriate dose of allopurinol of 200 mg, DUZALLO may be initiated by using one tablet of DUZALLO 200 mg lesinurad /200 mg allopurinol daily in place of 200 mg of allopurinol.
For patients currently on ZURAMPIC (lesinurad) in combination with allopurinol, DUZALLO may be initiated by using one tablet of DUZALLO in place of ZURAMPIC (lesinurad) and an equivalent portion of the daily allopurinol dose.
2.2 Patients With Renal Impairment
Patients with decreased renal function require lower doses of allopurinol than those with normal renal function. No dose adjustment is needed for lesinurad in patients with mild or moderate renal impairment (eCLcr of 45 mL/min or greater).
Assessment of renal function is recommended prior to initiation of DUZALLO and periodically thereafter [see Warnings and Precautions (5.1)]. DUZALLO should not be initiated in patients with an eCLcr less than 45 mL/min. DUZALLO should be discontinued when eCLcr is persistently less than 45 mL/min [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)]. More frequent renal function monitoring is recommended in patients with an eCLcr below 60 mL/min.
2.3 Gout Flares
Gout flares may occur after initiation of urate-lowering therapy, including DUZALLO, due to changing serum uric acid levels resulting in mobilization of urate from tissue deposits. For patients not currently taking lesinurad, gout flare prophylaxis is recommended when starting DUZALLO, according to practice guidelines.
If a gout flare occurs during DUZALLO treatment, DUZALLO need not be discontinued. The gout flare should be managed concurrently, as appropriate for the individual patient [see Patient Counseling Information (17)].
3. Dosage Forms and Strengths
DUZALLO is a combination of lesinurad and allopurinol. DUZALLO capsule-shaped tablets are available in the following dosage forms and strengths:
- 200 mg/200 mg tablets are light orange film-coated tablets debossed with "LES200" above "ALO200".
- 200 mg/300 mg tablets are dark orange film-coated tablets debossed with "LES200" above "ALO300".
4. Contraindications
The use of DUZALLO is contraindicated in the following conditions:
- Severe renal impairment (eCLcr less than 30 mL/min), end-stage renal disease, kidney transplant recipients, or patients on dialysis [see Use in Specific Populations (8.6)].
- Tumor lysis syndrome or Lesch-Nyhan syndrome [see Use in Specific Populations (8.8)].
- Known hypersensitivity to allopurinol, including previous occurrence of skin rash [see Warnings and Precautions (5.2)].
5. Warnings and Precautions
5.1 Renal Events
Adverse reactions related to renal function, including acute renal failure, can occur after initiating DUZALLO. Treatment with lesinurad 200 mg in combination with allopurinol was associated with an increased incidence of serum creatinine elevations, most of which were reversible [see Adverse Reactions (6.1)]. A higher incidence of serum creatinine elevations and renal-related adverse reactions, including serious adverse reactions of acute renal failure, were observed with lesinurad 400 mg in combination with allopurinol, with the highest incidence when lesinurad was given alone. DUZALLO treatment should be interrupted if serum creatinine is elevated to greater than 2 times the value when lesinurad treatment was initiated. In patients who report symptoms that may indicate acute uric acid nephropathy including flank pain, nausea or vomiting, interrupt treatment and measure serum creatinine promptly. DUZALLO should not be restarted without another explanation for the serum creatinine abnormalities.
DUZALLO should not be initiated in patients with an eCLcr less than 45 mL/min. Renal function should be evaluated prior to initiation of DUZALLO and periodically thereafter, as clinically indicated. More frequent renal function monitoring is recommended in patients with an eCLcr less than 60 mL/min [see Renal Impairment (8.6)] or with serum creatinine elevations 1.5 to 2 times the value when lesinurad treatment was initiated.
Some patients with pre-existing renal disease or poor urate clearance have shown a rise in BUN during administration of allopurinol.
5.2 Skin Rash and Hypersensitivity
Skin rash is a frequently reported adverse event in patients taking allopurinol [see Adverse Reactions (6.2)]. In some instances, a skin rash may be followed by more severe hypersensitivity reactions associated with exfoliation, fever, lymphadenopathy, arthralgia and/or eosinophilia including Stevens-Johnson syndrome and toxic epidermal necrolysis. Associated vasculitis and tissue response may be manifested in various ways including hepatitis, renal impairment, seizures, and on rare occasions, death. The HLA-B*5801 allele is a genetic risk marker for severe skin reactions indicative of hypersensitivity to allopurinol.
DUZALLO should be discontinued immediately at the first appearance of skin rash or other signs which may indicate an allergic reaction, and additional medical care should be provided as needed.
Hypersensitivity reactions to allopurinol may be increased in patients with decreased renal function receiving thiazide diuretics and DUZALLO concurrently. For this reason, in this clinical setting, such combinations should be administered with caution and patients should be observed closely.
5.3 Hepatotoxicity
A few cases of reversible clinical hepatotoxicity have been reported in patients taking allopurinol, and in some patients, asymptomatic rises in serum alkaline phosphatase or serum transaminase have been observed. If anorexia, weight loss, or pruritus develops in patients on DUZALLO, evaluation of liver function should be performed. In patients with pre-existing liver disease, periodic liver function tests are recommended.
5.4 Cardiovascular Events
In clinical trials with lesinurad and allopurinol, major adverse cardiovascular events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) were observed [see Adverse Reactions (6.1)]. A causal relationship has not been established.
5.5 Bone Marrow Depression
Bone marrow depression has been reported in patients receiving allopurinol, most of whom received concomitant drugs with the potential for causing this reaction. This has occurred as early as six weeks to as long as six years after the initiation of allopurinol therapy. Rarely a patient may develop varying degrees of bone marrow depression, affecting one or more cell lines, while receiving allopurinol alone.
Patients taking allopurinol and mercaptopurine or azathioprine require a reduction in dose to approximately one-third to one-fourth of the usual dose of mercaptopurine or azathioprine. Subsequent adjustment of doses of mercaptopurine or azathioprine should be made on the basis of therapeutic response and the appearance of toxic effects. Patients should be closely monitored for therapeutic response and the appearance of toxicity [see Drug Interaction (7.2)].
5.6 Increase in Prothrombin Time
It has been reported that allopurinol prolongs the half-life of dicumarol, a coumarin anticoagulant. The prothrombin time should be reassessed periodically in patients receiving coumarin anticoagulants (dicumarol, warfarin) concomitantly with DUZALLO [see Drug Interaction (7.2)].
5.7 Drowsiness
Occasional occurrence of drowsiness was reported in patients taking allopurinol. Patients should be alerted to the need for due caution when engaging in activities where alertness is mandatory [see Patient Counseling Information (17)].
6. Adverse Reactions/Side Effects
The following adverse reactions are also discussed in other sections:
- Renal Events [see Boxed Warning and Warnings and Precautions (5.1)].
- Skin Rash and Hypersensitivity [see Warnings and Precautions (5.2)].
- Hepatotoxicity [see Warnings and Precautions (5.3)].
- Cardiovascular Events [see Warnings and Precautions (5.4)].
- Bone Marrow Depression [see Warnings and Precautions (5.5)].
- Drowsiness [see Warnings and Precautions (5.7)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Lesinurad in Combination With Allopurinol
In 2 randomized, placebo-controlled studies (Studies 1 and 2), a total of 405, 401, and 407 patients with gout were treated with lesinurad 200 mg, lesinurad 400 mg, or placebo, respectively, once daily in combination with allopurinol (200 mg to 900 mg daily) for up to 12 months. The majority of patients in these studies received allopurinol daily doses of 200 mg or 300 mg (average dose of allopurinol in the studies was 310 mg), corresponding to the allopurinol doses contained in DUZALLO. The median duration of treatment with lesinurad in combination with allopurinol was 11.2 months. The mean age of the population was 52 years (range 18-82), and 95% were males. At baseline, 61% of the patient population showed mild or moderate renal impairment (eCLcr less than 90 mL/min) and 81% of patients had at least one co-morbid condition including hypertension (66%), hyperlipidemia (46%), diabetes (17%), and kidney stones (12%).
Renal Events
Lesinurad causes an increase in renal uric acid excretion, which may lead to renal events including transient increases in serum creatinine, renal-related adverse reactions, and kidney stones. These renal events occurred more frequently in patients receiving lesinurad 400 mg than in patients receiving 200 mg [see Warnings and Precautions (5.1)].
The number of patients with serum creatinine elevations in the 12-month placebo-controlled trials in combination with allopurinol is shown in Table 1. Most of these elevations on lesinurad 200 mg in combination with allopurinol and lesinurad 400 mg in combination with allopurinol resolved without treatment interruption (Table 1).
| [n (%)] | Placebo + Allopurinol (N=407) | Lesinurad 200 mg + Allopurinol (N=405) | Lesinurad 400 mg + Allopurinol (N=401) |
|---|---|---|---|
| Serum creatinine elevation 1.5 × to < 2.0 × baseline | 9 (2.2%) | 18 (4.4%) | 44 (11.0%) |
| Resolution of serum creatinine elevations by end of study | 6/9 (66.7%) | 16/18 (88.9%) | 35/44 (79.5%) |
| Serum creatinine elevation ≥ 2.0 × baseline | 0 | 6 (1.5%) | 28 (7.0%) |
| Resolution of serum creatinine elevations by end of study | N/A | 6/6 (100.0%) | 21/28 (75.0%) |
Renal-related adverse reactions, including blood creatinine increased and renal failure, and nephrolithiasis reported in patients receiving lesinurad 200 mg, lesinurad 400 mg, and placebo in combination with allopurinol are shown in Table 2 [see Warnings and Precautions (5.1)]. The incidence of reports of "blood creatinine increased" was higher with lesinurad in combination with allopurinol and was highest with lesinurad 400 mg in combination with allopurinol. Renal-related adverse reactions by baseline renal function category are shown in Table 3 [see Warnings and Precautions (5.1)]. Blood creatinine increased occurred more frequently in patients treated with lesinurad 400 mg in combination with allopurinol across baseline renal function categories (Table 3).
| [n (%)] | Placebo + Allopurinol (N=407) | Lesinurad 200 mg + Allopurinol (N=405) | Lesinurad 400 mg + Allopurinol (N=401) |
|---|---|---|---|
|
|||
| Blood creatinine increased | 9 (2.2%) | 15 (3.7%) | 32 (8.0%) |
| Renal failure* | 7 (1.7%) | 4 (1.0%) | 14 (3.5%) |
| Nephrolithiasis | 5 (1.2%) | 2 (0.5%) | 9 (2.2%) |
| n (%) | Placebo + Allopurinol | Lesinurad 200 mg + Allopurinol | Lesinurad 400 mg + Allopurinol |
|---|---|---|---|
|
|||
| ≥ 90 mL/min | n=149 | n=163 | n=161 |
| Blood creatinine increased | 0 | 5 (3.1%) | 6 (3.7%) |
| Renal failure* | 0 | 2 (1.2%) | 6 (3.7%) |
| ≥ 60 - < 90 mL/min | n=176 | n=167 | n=168 |
| Blood creatinine increased | 3 (1.7%) | 5 (3.0%) | 17 (10.1%) |
| Renal failure* | 4 (2.3%) | 1 (0.6%) | 6 (3.6%) |
| ≥ 30 - < 60 mL/min | n=78 | n=73 | n=70 |
| Blood creatinine increased | 6 (7.7%) | 4 (5.5%) | 8 (11.4%) |
| Renal failure* | 3 (3.8%) | 1 (1.4%) | 2 (2.9%) |
Renal-related adverse reactions resulted in a similar discontinuation rate on lesinurad 200 mg in combination with allopurinol (1.0%) and allopurinol alone (1.0%) and a higher rate on lesinurad 400 mg in combination with allopurinol (3.2%). Serious renal-related adverse reactions were reported in patients on lesinurad 400 mg in combination with allopurinol (0.7%) and allopurinol alone (0.2%) and in no patients on lesinurad 200 mg in combination with allopurinol during the 12-month controlled period of the studies. Serious renal-related adverse reactions were reported with lesinurad 200 mg and lesinurad 400 mg in combination with allopurinol in the uncontrolled long-term extensions [see Warnings and Precautions (5.1)].
Cardiovascular Safety
Cardiovascular events and deaths were adjudicated as Major Adverse Cardiovascular Events (cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) in the Phase 3 randomized controlled studies of lesinurad in combination with allopurinol. In the randomized controlled studies, the numbers of patients with adjudicated MACE (incidences per 100 patient-years of exposure) were: 2 (0.60) for placebo, 2 (0.61) for lesinurad 200 mg, and 6 (1.85) for lesinurad 400 mg when used in combination with allopurinol.
Other Adverse Reactions
Adverse reactions occurring in 2% or more of patients on lesinurad in combination with a xanthine oxidase inhibitor and at least 1% greater than that observed in patients on placebo with a xanthine oxidase inhibitor were identified in the lesinurad development program. The incidence of these adverse reactions in controlled studies with lesinurad 200 mg in combination with allopurinol is summarized in Table 4.
| Adverse Reaction | Placebo + Allopurinol (N=407) | Lesinurad 200 mg + Allopurinol (N=405) |
|---|---|---|
| Headache | 3.2% | 4.2% |
| Influenza | 2.9% | 4.9% |
| Gastroesophageal reflux disease | 0.5% | 3.2% |
6.2 Postmarketing Experience
Allopurinol
The following adverse reactions have been identified during post-approval use of allopurinol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most frequent adverse reaction to allopurinol is skin rash [see Warnings and Precautions (5.2)].
Most Common Adverse Reactions
Gastrointestinal disorders: diarrhea, nausea
Investigations: blood alkaline phosphatase increased, aspartate aminotransferase increased, alanine aminotransferase increased
Skin and subcutaneous tissue disorders: rash, rash maculo-papular
Less Common (<1%) Adverse Reactions
Blood and lymphatic system disorders: ecchymosis, thrombocytopenia, eosinophilia, leukocytosis, leukopenia
Gastrointestinal disorders: vomiting, abdominal pain, gastritis, dyspepsia
General disorders and administration site conditions: pyrexia
Hepatobiliary disorders: hepatitis (including hepatic necrosis and granulomatous hepatitis), hepatomegaly, hyperbilirubinemia, cholestatic jaundice
Musculoskeletal and connective tissue disorders: myopathy, arthralgia
Nervous system disorders: headache, peripheral neuropathy, neuritis, paresthesia, somnolence, ageusia (taste loss)
Renal and urinary disorders: renal failure, azotemia
Respiratory, thoracic and mediastinal disorders: epistaxis
Skin and subcutaneous tissue disorders: erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, leukocytoclastic vasculitis, purpura, bullous dermatitis, exfoliative dermatitis, eczema, pruritus, urticaria, alopecia, onycholysis, lichen planus
Vascular disorders: vasculitis necrotizing, vasculitis
Other Uncommon (<1%) Adverse Events
Blood and lymphatic system disorders: aplastic anemia, agranulocytosis, pancytopenia, anemia, hemolytic anemia, reticulocytosis, lymphadenopathy, lymphocytosis
Cardiac disorders: pericarditis, bradycardia
Endocrine disorders: hypercalcemia, gynecomastia
Eye disorders: cataract, retinitis, iritis, conjunctivitis, amblyopia
Gastrointestinal disorders: hemorrhagic pancreatitis, gastrointestinal hemorrhage, stomatitis, salivary gland enlargement, tongue edema
General disorders and administration site conditions: malaise, asthenia, hyperhidrosis
Infections and infestations: pharyngitis, rhinitis
Investigations: prothrombin level decreased
Metabolism and nutrition disorders: hyperlipidemia, decreased appetite
Musculoskeletal and connective tissue disorders: myalgia
Nervous system disorders: optic neuritis, confusional state, dizziness, vertigo, peroneal nerve palsy, amnesia, tinnitus, insomnia
Psychiatric disorders: libido decreased, depression, erectile dysfunction
Renal and urinary disorders: nephritis, hematuria, albuminuria
Reproductive system and breast disorders: infertility male
Respiratory, thoracic and mediastinal disorders: bronchospasm, asthma
Skin and subcutaneous tissue disorders: furuncle, face edema, skin edema
Vascular disorders: peripheral vascular disorder, thrombophlebitis, vasodilatation
7. Drug Interactions
No drug interactions studies were conducted with DUZALLO. However, as DUZALLO contains lesinurad and allopurinol, any interactions that have been identified with these agents individually may occur with DUZALLO. The following interactions have been noted with the individual components of DUZALLO.
7.1 Drug Interactions With Lesinurad
CYP2C9 Inhibitors, CYP2C9 Poor Metabolizers, and CYP2C9 Inducers
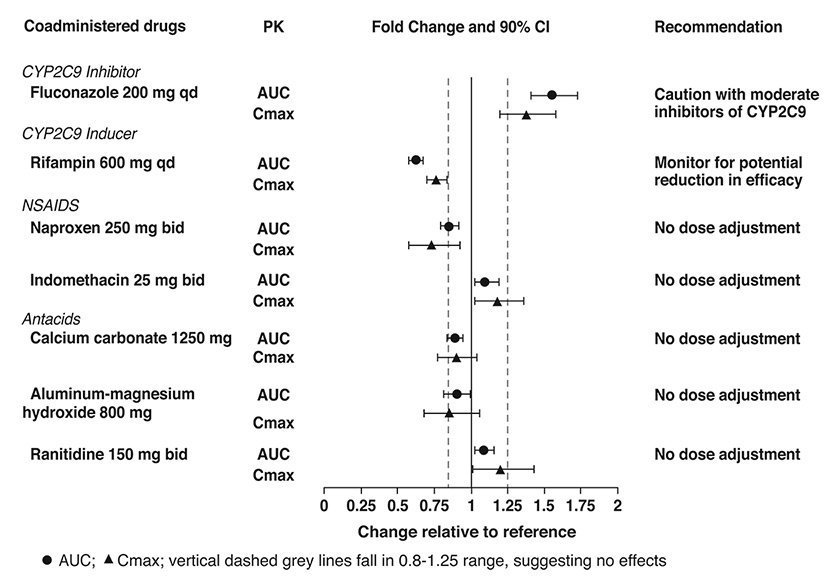
Co-administration of lesinurad with inhibitors of CYP2C9, and administration of lesinurad to CYP2C9 poor metabolizers, resulted in increased lesinurad exposure. DUZALLO should be used with caution in patients taking moderate inhibitors of CYP2C9 (e.g., fluconazole, amiodarone), and in CYP2C9 poor metabolizers [see Clinical Pharmacology (12.3)].
Lesinurad exposure is decreased when lesinurad is co-administered with moderate inducers of CYP2C9 (e.g., rifampin, carbamazepine), which may decrease the therapeutic effect of DUZALLO [see Clinical Pharmacology (12.3)].
CYP3A Substrates
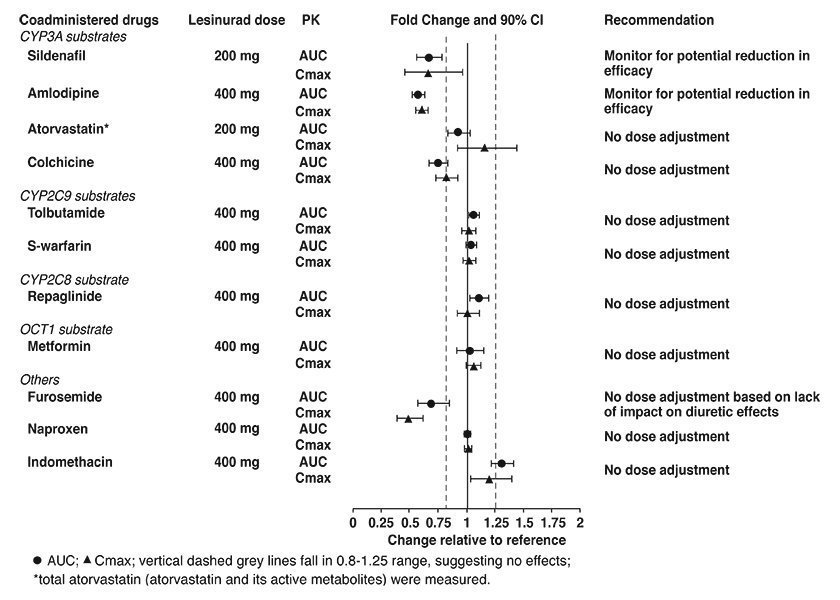
In interaction studies conducted in healthy subjects with lesinurad and CYP3A substrates, lesinurad reduced the plasma concentrations of sildenafil and amlodipine [see Clinical Pharmacology (12.3)]. Although there was not a clinically significant interaction with atorvastatin, HMG-CoA reductase inhibitors that are sensitive CYP3A substrates may be affected by co-administration with DUZALLO. The possibility of reduced efficacy of concomitant drugs that are CYP3A substrates should be considered and their efficacy (e.g., blood pressure and cholesterol levels) should be monitored.
Epoxide Hydrolase Inhibitors
In vitro studies suggest that lesinurad is not an inhibitor of epoxide hydrolase; however, inhibitors of epoxide hydrolase (i.e., valproic acid) may interfere with metabolism of lesinurad. DUZALLO should not be administered with inhibitors of epoxide hydrolase.
7.2 Drug Interactions With Allopurinol
Immunosuppressants/Cytotoxics
Mercaptopurine and azathioprine
Azathioprine is metabolized to mercaptopurine which is inactivated by the action of xanthine oxidase. Since allopurinol is an inhibitor of xanthine oxidase, the activity of azathioprine and mercaptopurine is prolonged. Therefore, during concomitant administration with DUZALLO, the dose of mercaptopurine or azathioprine will require reduction to approximately one-third to one-fourth of the usual dose, and should be closely monitored for therapeutic response and the appearance of toxicity. Subsequent adjustment of doses of mercaptopurine or azathioprine should be made on the basis of therapeutic response and the appearance of toxic effects [see Warnings & Precautions (5.5) and Clinical Pharmacology (12.3)].
Cyclophosphamide, doxorubicin, bleomycin, procarbazine, mechloroethamine
Enhanced bone marrow suppression by cyclophosphamide and other cytotoxic agents has been reported among patients with neoplastic disease (except leukemia), in the presence of allopurinol. However, in a well-controlled study of patients with lymphoma on combination therapy, allopurinol did not increase the marrow toxicity of patients treated with cyclophosphamide, doxorubicin, bleomycin, procarbazine, and/or mechlorethamine [see Warnings and Precautions (5.5)].
Dicumarol
There have been reports of prolonged half-life of dicumarol, a coumarin anticoagulant, when coadministered with allopurinol; therefore, patients receiving DUZALLO in addition to dicumarol must be carefully monitored [see Warnings & Precautions (5.6)].
Thiazide diuretics
The concomitant use of allopurinol and thiazide diuretics may contribute to the enhancement of allopurinol toxicity in some patients. Although a causal mechanism and a cause-and-effect relationship have not been established, current evidence suggests that renal function should be monitored in patients on thiazide diuretics and allopurinol even in the absence of renal failure, and dosage levels should be even more conservatively adjusted in those patients on such combined therapy if diminished renal function is detected [see Warnings and Precautions (5.2)].
Ampicillin/Amoxicillin
An increase in the frequency of skin rash has been reported among patients receiving amoxicillin or ampicillin concurrently with allopurinol compared to patients who are not receiving both drugs. The cause of the association has not been established. [see Warnings and Precautions (5.2)].
Chlorpropamide
Plasma half-life of chlorpropamide may be prolonged by allopurinol, since allopurinol and chlorpropamide may compete for excretion in the renal tubule. The risk of hypoglycemia secondary to this mechanism may be increased if allopurinol and chlorpropamide are given concomitantly in the presence of renal insufficiency.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available human data on use of DUZALLO or lesinurad in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Limited published data on allopurinol use in pregnant women do not demonstrate a clear pattern or increase in frequency of adverse development outcomes.
Animal reproductive toxicity studies have not been conducted with DUZALLO; however, studies were conducted with its individual components [see Animal Data]. No teratogenicity or effects on fetal development were observed in embryo-fetal development studies with oral administration of lesinurad to pregnant rats and rabbits during organogenesis at doses that produced maternal exposures up to 45 and 10 times, respectively, the exposure at the maximum recommended human dose (MRHD). No teratogenic or fetotoxic effects were observed in embryo-fetal development studies with orally administered allopurinol to pregnant rats and rabbits during organogenesis at doses approximately 6 times the MRHD in both species. However, intraperitoneal administration of allopurinol to pregnant mice on gestation days 10 or 13, during the period of organogenesis, produced fetal deaths and teratogenic effects at doses 0.8 times the MRHD and higher.
No adverse developmental effects were observed in a pre- and post-natal development study with oral administration of lesinurad to pregnant rats from organogenesis through lactation at a dose approximately 5 times the MRHD.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
Lesinurad
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6-17, lesinurad was not teratogenic and did not affect fetal development or survival at exposures up to approximately 45 times the MRHD (on an AUC basis at maternal oral doses up to 300 mg/kg/day). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 7-20, lesinurad was not teratogenic and did not affect fetal development at exposures up to approximately 10 times the MRHD (on an AUC basis at maternal oral doses up to 75 mg/kg/day). Severe maternal toxicity, including mortality, was observed in rats and rabbits at exposures equal to or greater than approximately 45 and 4 times the MRHD (on an AUC basis at maternal oral doses of 300 mg/kg/day in rats and 25 mg/kg/day and higher in rabbits) respectively.
In a pre- and post-natal development study in pregnant female rats dosed from gestation day 7 through lactation day 20, lesinurad had no effects on delivery or growth and development of offspring at a dose approximately 5 times the MRHD (on a mg/m2 basis at a maternal oral dose of 100 mg/kg/day). In rats, plasma and milk concentrations of lesinurad were approximately equal.
Allopurinol
In embryo-fetal development studies with pregnant rats or rabbits, allopurinol administered during the period of organogenesis (gestation days 8 – 16) was not teratogenic or fetotoxic in either species at doses up to approximately 6 times the MRHD (on a mg/m2 basis at maternal oral doses up to 200 mg/kg/day in rats and 100 mg/kg/day in rabbits). However, in an embryo-fetal development study with pregnant mice, single intraperitoneal doses of allopurinol on gestation days 10 or 13 produced significant increases in fetal deaths and teratogenic effects, including external and skeletal malformations, at 0.8 times the MRHD and higher (on a mg/m2 basis at maternal intraperitoneal doses of 50 or 100 mg/kg). It is uncertain whether the findings in mice represented a fetal effect or an effect secondary to maternal toxicity.
Allopurinol crossed the placental barrier following oral administration to pregnant pigs and was detected in fetal plasma.
8.2 Lactation
Risk Summary
There is no information regarding the presence of DUZALLO or lesinurad in human milk, the effects on the breastfed infant, or the effects on milk production. Lesinurad was present in the milk of rats [see Use in Specific Populations (8.1)]. Based on information from a single case report, allopurinol and its active metabolite, oxypurinol, were detected in the milk of a mother at five weeks postpartum. There was no report of effects of allopurinol on the breastfed infant or on milk production. The effect of allopurinol on the nursing infant is unknown.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DUZALLO and any potential adverse effects on the breastfed infant from DUZALLO or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
No dose adjustment is necessary in elderly patients. In a pool of clinical safety and efficacy studies of lesinurad in combination with allopurinol in gout patients, 12% were 65 years and older and 2% were 75 years and older. No overall differences between lesinurad in combination with allopurinol and allopurinol alone in safety and effectiveness were observed between these subjects and younger subjects. Greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
The efficacy and safety of lesinurad in combination with allopurinol were evaluated in studies that included gout patients with mild and moderate renal impairment [see Adverse Reactions (6.1) and Clinical Studies (14)]. There were no clear differences in safety and effectiveness of lesinurad in combination with allopurinol in patients with mild renal impairment compared to patients with normal renal function. No lesinurad dose adjustment is recommended [see Dosage and Administration (2.2), and Clinical Studies (14.3)].
Across all lesinurad and placebo treatment groups, patients with moderate renal impairment had a higher occurrence of renal-related adverse reactions compared to patients with mild renal impairment or normal renal function [see Adverse Reactions (6.1)]. The experience with lesinurad in combination with allopurinol in patients with an eCLcr less than 45 mL/min is limited and there was a trend toward lesser efficacy [see Clinical Studies (14.3)]. DUZALLO should not be initiated in patients with an eCLcr less than 45 mL/min. No lesinurad dose adjustment is recommended in patients with an eCLcr 45 to less than 60 mL/min, however, more frequent renal function monitoring is recommended. DUZALLO should be discontinued when eCLcr is persistently less than 45 mL/min [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
The efficacy and safety of DUZALLO have not been evaluated in gout patients with severe renal impairment (eCLcr less than 30 mL/min), with end-stage renal disease, or receiving dialysis. DUZALLO is not expected to be effective in these patient populations [see Contraindications (4)].
Lesinurad
The pharmacokinetics (PK) of lesinurad were evaluated in studies that included patients with mild (eCLcr 60 to less than 90 mL/min), moderate (eCLcr 30 to less than 60 mL/min), and severe renal impairment (eCLcr less than 30 mL/min). Lesinurad exposure (AUC) increased by 30%, 50-73%, and 113%, respectively, in subjects with mild, moderate, and severe renal impairment [see Clinical Pharmacology (12.3)].
Allopurinol
Allopurinol and its metabolites are excreted via the kidney. Impairment of renal function may lead to retention of the drug and its metabolites with consequent prolongation of action. In patients with severely impaired renal function or decreased urate clearance, the half-life of oxypurinol in the plasma is greatly prolonged.
Patients with reduced renal function require lower doses of allopurinol than those with normal renal function [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh classes A and B) [see Clinical Pharmacology (12.3)]. Neither DUZALLO nor its individual components have been studied in patients with severe hepatic impairment; DUZALLO is therefore not recommended in these patients [see Warnings and Precautions (5.3)].
8.8 Secondary Hyperuricemia
No studies with DUZALLO have been conducted in patients with secondary hyperuricemia (including organ transplant recipients); DUZALLO is contraindicated for use in tumor lysis syndrome or Lesch-Nyhan syndrome, where the rate of uric acid formation is greatly increased [see Contraindications (4)].
10. Overdosage
Lesinurad
Lesinurad was studied in healthy subjects given single doses up to 1600 mg without evidence of dose-limiting toxicities. In case of overdose patients should be managed by symptomatic and supportive care including adequate hydration. The removal of lesinurad by hemodialysis has not been studied.
Allopurinol
There are published reports of allopurinol overdose in humans, with ingestion of doses up to 22.5 grams. Symptoms and signs including nausea, vomiting, diarrhea and dizziness have been reported. In the management of overdosage there is no specific antidote for allopurinol. Both allopurinol and oxypurinol are dialyzable; however, the usefulness of hemodialysis or peritoneal dialysis in the management of an overdose of allopurinol is unknown.
11. Duzallo Description
DUZALLO (lesinurad and allopurinol) tablets contain 2 oral medications used in the treatment of hyperuricemia: lesinurad and allopurinol. Lesinurad is a URAT1 inhibitor and allopurinol is a xanthine oxidase inhibitor.
Lesinurad
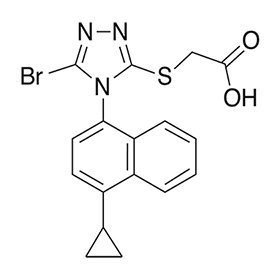
Lesinurad has the following chemical name: 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetic acid. The molecular formula is C17H14BrN3O2S and the molecular weight is 404.28. The structural formula is:
Allopurinol
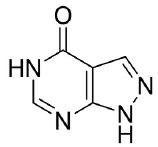
Allopurinol has the following chemical name: 1,5-dihydro-4H-Pyrazolo[3,4-d]pyrimidin-4-one. The molecular formula is C5H4N4O and the molecular weight is 136.11. The structural formula is:
DUZALLO
DUZALLO is available as a light orange film-coated tablet containing 200 mg of lesinurad and 200 mg of allopurinol or a dark orange film-coated tablet containing 200 mg of lesinurad and 300 mg of allopurinol. The capsule-shaped tablets include the following inactive ingredients: crospovidone, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: hypromellose, iron oxide red, iron oxide yellow, titanium dioxide, and triacetin. DUZALLO 200/200 mg tablets are coated with Opadry orange and 200/300 mg tablets are coated with Opadry beige.
12. Duzallo - Clinical Pharmacology
12.1 Mechanism of Action
DUZALLO combines two medications with complementary mechanisms of action for treatment of hyperuricemia associated with gout: lesinurad, a uric acid reabsorption inhibitor, and allopurinol, a xanthine oxidase inhibitor. DUZALLO lowers serum uric acid levels by increasing excretion and inhibiting production of uric acid.
Lesinurad
Lesinurad reduces serum uric acid levels by inhibiting the function of transporter proteins involved in uric acid reabsorption in the kidney. Lesinurad inhibited the function of two apical transporters responsible for uric acid reabsorption, uric acid transporter 1 (URAT1) and organic anion transporter 4 (OAT4), with IC50 values of 7.3 and 3.7 µM, respectively. URAT1 is responsible for the majority of the reabsorption of filtered uric acid from the renal tubular lumen. OAT4 is a uric acid transporter associated with diuretic-induced hyperuricemia. Lesinurad does not interact with the uric acid reabsorption transporter SLC2A9 (Glut9), located on the basolateral membrane of the proximal tubule cell.
Allopurinol
Allopurinol acts on purine catabolism, without disrupting the biosynthesis of purines. It reduces the production of uric acid by inhibiting the biochemical reactions immediately preceding its formation. Allopurinol is a structural analogue of the natural purine base, hypoxanthine. It is an inhibitor of xanthine oxidase, the enzyme responsible for the conversion of hypoxanthine to xanthine and of xanthine to uric acid, the end product of purine metabolism in humans. Allopurinol is metabolized to the corresponding xanthine analogue, oxypurinol (alloxanthine), which also is an inhibitor of xanthine oxidase.
Reutilization of both hypoxanthine and xanthine for nucleotide and nucleic acid synthesis is markedly enhanced when their oxidations are inhibited by allopurinol and oxypurinol. This reutilization does not disrupt normal nucleic acid anabolism because feedback inhibition is an integral part of purine biosynthesis. As a result of xanthine oxidase inhibition, the serum concentration of hypoxanthine plus xanthine in patients receiving allopurinol for treatment of hyperuricemia is usually in the range of 0.3 to 0.4 mg/dL compared to a normal level of approximately 0.15 mg/dL. A maximum of 0.9 mg/dL of these oxypurines has been reported when the serum urate was lowered to less than 2 mg/dL by high doses of allopurinol. These values are far below the saturation levels at which point their precipitation would be expected to occur (above 7 mg/dL).
12.2 Pharmacodynamics
Effects on Serum Uric Acid and Urinary Excretion of Uric Acid
Lesinurad
In gout patients, lesinurad lowered serum uric acid levels and increased renal clearance and fractional excretion of uric acid. Following single and multiple oral doses of lesinurad to gout patients, dose-dependent decreases in serum uric acid levels and increases in urinary uric acid excretion were observed.
12.3 Pharmacokinetics
DUZALLO
After the administration of DUZALLO with a high-fat breakfast in healthy subjects, the exposure (AUC) for lesinurad and allopurinol were comparable to the fasted state. Compared to the fasted state, the fed state resulted in 46% and 18% reductions and 2.5 hours and 1.75 hours delays, in the peak (Cmax) concentrations of lesinurad and allopurinol, respectively. This effect of food is not considered to be clinically meaningful. Oxypurinol Cmax and AUC were similar in the fed and fasted states.
Absorption
Lesinurad
The absolute bioavailability of lesinurad as monotherapy is approximately 100%. Lesinurad is rapidly absorbed after oral administration. Following administration of a single dose of a lesinurad tablet in either fed or fasted state, Cmax was attained within 1 to 4 hours. Cmax and AUC exposures of lesinurad increased proportionally with single doses of lesinurad from 5 to 1200 mg. Administration with a high-fat meal (800 to 1000 calories with 50% of calories being derived from fat content) decreases Cmax by up to 18% but does not alter AUC as compared with fasted state. In clinical trials, lesinurad was administered with food.
Allopurinol
Allopurinol is approximately 90% absorbed from the gastrointestinal tract. Peak plasma levels generally occur at 1.5 hours and 4.5 hours for allopurinol and oxypurinol, respectively. After a single oral dose of 300 mg allopurinol, maximum plasma levels of about 3 mcg/mL of allopurinol and 6.5 mcg/mL of oxypurinol are produced.
Distribution
Lesinurad
Lesinurad is extensively bound to proteins in plasma (greater than 98%), mainly to albumin. Plasma protein binding of lesinurad is not meaningfully altered in patients with renal or hepatic impairment. The mean steady state volume of distribution of lesinurad was approximately 20 L following intravenous dosing of lesinurad.
Elimination
Lesinurad
The elimination half-life (t½) of lesinurad is approximately 5 hours. Lesinurad does not accumulate following multiple doses. The total body clearance is approximately 6 L/hr.
Allopurinol
Because of its rapid oxidation to oxypurinol and a renal clearance rate approximately that of glomerular filtration rate, allopurinol has a plasma half-life of about 1 to 2 hours. Oxypurinol, however, has a longer plasma elimination half-life (approximately 26 hours) and therefore effective xanthine oxidase inhibition is maintained over a 24-hour period with single daily doses of allopurinol. Whereas allopurinol is cleared essentially by glomerular filtration, oxypurinol is reabsorbed in the kidney tubules in a manner similar to the reabsorption of uric acid.
Metabolism
Lesinurad
Lesinurad undergoes oxidative metabolism mainly via the polymorphic cytochrome P450 CYP2C9 enzyme. Plasma exposure of metabolites is minimal (<10% of unchanged lesinurad). Metabolites are not known to contribute to the uric acid lowering effects of lesinurad. A transient oxide metabolite is rapidly eliminated by microsomal epoxide hydrolase in the liver and not detected in plasma.
Patients who are CYP2C9 poor metabolizers are deficient in CYP2C9 enzyme activity. A cross-study pharmacogenomic analysis assessed the association between CYP2C9 polymorphism and lesinurad exposure in patients receiving single or multiple doses of lesinurad at 200 mg, 400 mg or 600 mg. At the 400 mg dose, lesinurad exposure was approximately 1.8-fold higher in CYP2C9 poor metabolizers (i.e., subjects with CYP2C9 *2/*2 [N=1], and *3/*3 [N=1] genotype) compared to CYP2C9 extensive metabolizers (i.e., CYP2C9 *1/*1 [N=41] genotype). Use with caution in CYP2C9 poor metabolizers, and in patients taking moderate inhibitors of CYP2C9 [see Drug Interactions (7.1)].
Excretion
Lesinurad
Within 7 days following single dosing of radiolabeled lesinurad, 63% of administered radioactive dose was recovered in urine and 32% of administered radioactive dose was recovered in feces. Most of the radioactivity recovered in urine (> 60% of dose) occurred in the first 24 hours. Unchanged lesinurad in urine accounted for approximately 30% of the dose.
SPECIFIC POPULATIONS
Renal Impairment
A dedicated renal impairment study was not conducted with DUZALLO.
Lesinurad
Two dedicated studies were performed to assess pharmacokinetics (PK) of lesinurad in renal impairment (classified using the Cockcroft-Gault formula) subjects. In both studies, Cmax was comparable in renal impairment subjects compared to healthy subjects. Study 1 was a single-dose, open-label study evaluating the PK of lesinurad 200 mg in subjects with mild (eCLcr 60 to less than 90 mL/min) and moderate renal impairment (eCLcr 30 to less than 60 mL/min) compared to healthy subjects. Compared to healthy subjects (N=6; eCLcr greater than or equal to 90 mL/min), plasma AUC of lesinurad was increased by approximately 30% and 73% in subjects with mild (N=8) and moderate (N=10) renal impairment, respectively. Study 2 was a single-dose, open-label study evaluating the PK of lesinurad 400 mg in subjects with moderate and severe renal impairment (eCLcr less than 30 mL/min) compared to healthy subjects. Compared to healthy subjects (N=6), plasma AUC of lesinurad was increased by approximately 50% and 113% in subjects with moderate (N=6) and severe (N=6) renal impairment, respectively.
Allopurinol
Allopurinol and its metabolites are primarily eliminated by the kidney. Impairment of renal function may lead to retention of the drug and its metabolites with consequent prolongation of action. Patients with reduced renal function require lower doses of allopurinol than those with normal renal function.
Hepatic Impairment
A dedicated hepatic impairment study was not conducted with DUZALLO.
Lesinurad
Following administration of a single dose of lesinurad at 400 mg in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, lesinurad Cmax was comparable and lesinurad AUC was 7% and 33% higher, respectively, compared to individuals with normal hepatic function. There is no clinical experience in patients with severe (Child-Pugh class C) hepatic impairment.
Allopurinol
No information is available in patients with hepatic impairment [see Warnings and Precautions (5.3)].
Effect of Age, Gender, Race and Ethnicity on Pharmacokinetics
Lesinurad
Based on the population PK analysis, age, gender, race and ethnicity do not have a clinically meaningful effect on the PK of lesinurad [see Use in Specific Populations (8.5)].
Drug-Drug Interactions
Pharmacokinetic drug interaction studies with DUZALLO have not been performed; however, such studies have been conducted with the individual components lesinurad and allopurinol.
Lesinurad
Based on in vitro data, lesinurad is a substrate for CYP2C9, OAT1 and OAT3; however, no clinical studies have been conducted with OAT1 and OAT3 inhibitors (e.g., probenecid).
Effects of Other Drugs on Lesinurad
Figure 1 shows the effect of co-administered drugs on the PK of lesinurad.
Figure 1: Effect of Co-Administered Drugs on the Pharmacokinetics of Lesinurad
Effects of Lesinurad on Other Drugs
Lesinurad is a weak inducer of CYP3A and has no relevant effect on any other CYP enzyme for induction (CYP1A2, CYP2C8, CYP2C9, CYP2B6, or CYP2C19) or inhibition (CYP1A2, CYP2B6, CYP2D6, CYP2C8, CYP2C9, CYP2C19, or CYP3A4).
Based on in vitro studies, lesinurad is an inhibitor of OATP1B1, OCT1, OAT1, and OAT3; however, lesinurad is not an in vivo inhibitor of these transporters. In vivo drug interaction studies indicate that lesinurad does not decrease the renal clearance of furosemide (substrate of OAT1/3), or affect the exposure of atorvastatin (substrate of OATP1B1) or metformin (substrate of OCT1). Based on in vitro studies, lesinurad has no relevant effect on P-glycoprotein.
Figure 2 shows the effect of lesinurad on co-administered drugs.
Figure 2: Effect of Lesinurad on the Pharmacokinetics of Coadministered Drugs
Effect of Allopurinol on Mercaptopurine
Allopurinol inhibits the enzymatic oxidation of mercaptopurine, the sulfur-containing analogue of hypoxanthine, to 6-thiouric acid. This oxidation, which is catalyzed by xanthine oxidase, inactivates mercaptopurine. Hence, the inhibition of such oxidation by allopurinol may result in as much as a 75% reduction in the therapeutic dose requirement of mercaptopurine when the two compounds are given together.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with DUZALLO; however, studies were conducted with its individual components, lesinurad and allopurinol, as described below.
Lesinurad
The carcinogenic potential of lesinurad was evaluated in a 2-year study conducted in Sprague-Dawley rats and a 26-week study in TgRasH2 mice. No evidence of tumorigenicity was observed in male or female rats at oral doses up to 200 mg/kg/day (approximately 35 times the MRHD on an AUC basis). No evidence of tumorigenicity was observed in TgRasH2 mice at oral doses up to 125 and 250 mg/kg/day in male and female mice, respectively.
Lesinurad tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo rat bone marrow micronucleus assay.
Fertility and reproductive performance were unaffected in male or female rats that received lesinurad at oral doses up to 300 mg/kg/day (approximately 15 times the MRHD on a mg/m2 basis).
Allopurinol
No evidence of tumorigenicity was observed in male or female mice or rats that received oral allopurinol for the majority of their life spans (greater than 88 weeks) at doses up to 20 mg/kg/day (0.3 and 0.6 times the MRHD on a mg/m2 basis in mice and rats respectively).
Allopurinol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo rat bone marrow micronucleus assay.
Oral allopurinol doses of 20 mg/kg/day had no effect on male or female fertility in rats or rabbits (approximately 0.6 or 1.3 times the MRHD on a mg/m2 basis, respectively).
14. Clinical Studies
Lesinurad in combination with allopurinol has been studied in hyperuricemic gout patients who have not achieved target serum uric acid levels with allopurinol alone.
There have been no phase 3 clinical trials with DUZALLO. Bioequivalence of DUZALLO to co-administered lesinurad and allopurinol was demonstrated, and efficacy of the combination of allopurinol and lesinurad has been demonstrated in two phase 3 studies (Study 1 and 2).
14.1 Lesinurad Add-On to Allopurinol in Inadequate Responders
Both studies were 12-month multicenter, randomized, double-blind, placebo-controlled clinical studies in adult patients with hyperuricemia and gout. Patients received prophylaxis for gout flares with colchicine or non-steroidal anti-inflammatory drugs (NSAIDs) during the first 5 months of study treatment. Lesinurad 200 mg and 400 mg once daily in combination with allopurinol were studied. Lesinurad 200 mg is the only recommended lesinurad dose, and is the dose combined with allopurinol in DUZALLO.
Study 1 and Study 2 enrolled patients with gout who were on a stable dose of allopurinol of at least 300 mg (or 200 mg for moderate renal impairment), had a serum uric acid > 6.5 mg/dL, and reported at least 2 gout flares in the prior 12 months. Mean years since gout diagnosis were 12 years. More than half of the patients (61%) had mild or moderate renal impairment and 19% of the patients had tophi. Patients were randomized 1:1:1 to receive lesinurad 200 mg, lesinurad 400 mg, or placebo once daily; all were to continue on their stable allopurinol dose. The majority of patients in these studies received daily allopurinol doses of 200 mg or 300 mg, corresponding to the allopurinol doses contained in DUZALLO. The average dose of allopurinol in the studies was 310 mg (range: 200-900 mg).
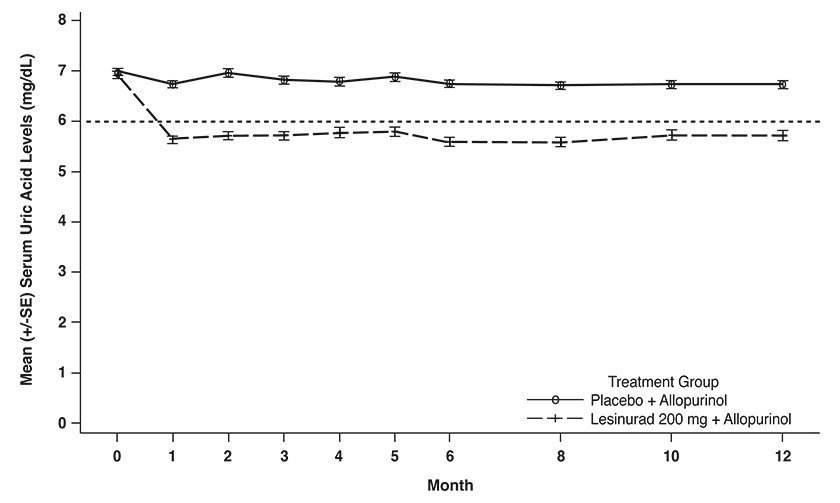
As shown in Table 5, lesinurad 200 mg in combination with allopurinol was superior to allopurinol alone in lowering serum uric acid to less than 6 mg/dL at Month 6.
| Study | Timepoint | Patients Achieving Serum Uric Acid Target | Difference of Proportion (95% C.I.) |
|
|---|---|---|---|---|
| Placebo + Allopurinol | Lesinurad 200 mg + Allopurinol | |||
|
||||
| Study 1 (N=402*) | Month 6 | 28% | 54% | 0.26 ( 0.17, 0.36) |
| Study 2 (N=410*) | Month 6 | 23% | 55% | 0.32 (0.23, 0.41) |
The estimated effect of lesinurad 200 mg on serum uric acid in the subgroup of patients taking thiazide diuretics at baseline was similar to the estimated effect in the overall population. The estimated effect was also similar in the subgroup of patients taking low-dose aspirin at baseline.
As shown in Figure 3, reduction in average serum uric acid levels to < 6 mg/dL was noted for lesinurad 200 mg in combination with allopurinol at the Month 1 visit and was maintained throughout the 12-month studies.
Figure 3: Mean Serum Uric Acid Levels Over Time in Pooled Clinical Studies With Lesinurad in Combination With Allopurinol (Study 1 and Study 2)
14.2 Gout Flares
In Study 1 and Study 2, the rates of gout flare requiring treatment from the end of Month 6 to the end of Month 12 were not statistically different between lesinurad 200 mg in combination with allopurinol compared with allopurinol alone.
14.3 Use in Patients With Renal Impairment
The estimated differences between lesinurad and placebo in the proportions of patients achieving target serum uric acid levels in the renal impairment subgroups were largely consistent with the results in the overall population in the studies. However, there were limited data in patients with eCLcr less than 45 mL/min and there was a trend toward decreasing magnitude of effect with decreasing renal function. Based on integrated data from Study 1 and Study 2, the estimated difference between lesinurad 200 mg in combination with allopurinol and allopurinol alone in the proportion achieving serum uric acid < 6.0 mg/dL at Month 6 was 10% (95% CI: -17, 37) in those with eCLcr less than 45 mL/min as compared with 27% (95% CI: 9, 45) in the 45 to less than 60 mL/min subgroup and 30% (95% CI: 23, 37) in the 60 mL/min or greater subgroup.
16. How is Duzallo supplied
16.1 How Supplied
DUZALLO tablets have markings on one side, are plain on the reverse side and are available in the strengths and packages listed in Table 6.
| Tablet Strength (Lesinurad/Allopurinol) | Capsule-Shaped, Film-Coated, Tablet | Tablet Markings | Pack Size | NDC Code |
|---|---|---|---|---|
| 200/200 mg | Light orange film-coated tablet | Debossed with "LES200" above "ALO200" | Bottle of 5 tablets Bottle of 30 tablets Bottle of 90 tablets | 70785-021-05 70785-021-30 70785-021-90 |
| 200/300 mg | Dark orange film-coated tablet | Debossed with "LES200" above "ALO300" | Bottle of 5 tablets Bottle of 30 tablets Bottle of 90 tablets | 70785-022-05 70785-022-30 70785-022-90 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration
Advise patients:
- To take one tablet of DUZALLO in the morning with food and water.
- Not to take a missed dose of DUZALLO later in the day, but to wait to take DUZALLO on the next day, and not to double the dose.
- Not to take DUZALLO with ZURAMPIC (lesinurad).
- To stay well hydrated (e.g., 2 liters of liquid per day).
Renal Events
Inform patients that renal events including transient increases in blood creatinine level and acute renal failure have occurred in some patients who take DUZALLO. Advise patients that periodic monitoring of blood creatinine levels is recommended [see Warnings and Precautions (5.1)].
Serious Skin Reactions
Inform patients that DUZALLO may increase the risk of serious skin side effects such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching and should ask for medical advice when observing any indicative signs or symptoms. Advise patients to stop the drug immediately if they develop any type of rash and seek medical attention [see Warnings and Precautions (5.2)].
Gout Flares
Inform patients that gout flares may occur after initiation of DUZALLO and of the importance of taking gout flare prophylaxis medication to help prevent gout flares. Advise patients not to discontinue DUZALLO if a gout flare occurs during treatment [see Dosage and Administration (2.3)].
Concomitant Use with Other Medications
Inform patients that use of DUZALLO may increase the risks associated with taking other medications (i.e., coumarin anticoagulants, mercaptopurine, azathioprine and thiazide diuretics) and they should follow the instructions of their healthcare provider [see Warnings and Precautions (5.5, 5.6), Drug Interactions (7)].
Ability to Perform Complex Tasks
Patients should be informed that drowsiness has been reported in patients taking allopurinol. Patients should be alerted to the need for due caution when engaging in activities where alertness is mandatory [see Warnings and Precautions (5.7)].
Manufactured for: Ironwood Pharmaceuticals, Inc., Cambridge, MA 02142
By: AstraZeneca AB, SE-151 85 Sodertalje, Sweden
Product of Sweden
Ironwood and the three-leaf design are registered trademarks of Ironwood Pharmaceuticals, Inc.
DUZALLO and ZURAMPIC are registered trademarks of the AstraZeneca group of companies.
© AstraZeneca and Ironwood Pharmaceuticals, Inc. 2017
| MEDICATION GUIDE DUZALLO® (Dew-ZAL-oh) (lesinurad and allopurinol) tablets, for oral use |
|||
|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: 11/2017 | ||
|
What is the most important information I should know about DUZALLO? DUZALLO can cause serious side effects, including:
|
|||
|
|
||
| See "What are the possible side effects of DUZALLO?" for more information about side effects. | |||
|
What is DUZALLO?
It is not known if DUZALLO is safe and effective in children under 18 years of age. |
|||
|
Do not take DUZALLO if you have:
|
|||
|
Before taking DUZALLO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. DUZALLO may affect the way other medicines work, and other medicines may affect how DUZALLO works. Especially tell your healthcare provider if you take:
Ask your healthcare provider or pharmacist if you are not sure if you take any of these medicines. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine. |
|||
|
How should I take DUZALLO?
|
|||
|
What should I avoid while taking DUZALLO?
|
|||
|
What are the possible side effects of DUZALLO? DUZALLO may cause serious side effects including:
|
|||
|
|
||
The most common side effects of DUZALLO include: |
|||
|
|
||
| Tell your healthcare provider if you have any side effect that bothers you, or that does not go away. These are not all of the possible side effects of DUZALLO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
How should I store DUZALLO?
Keep DUZALLO and all medicines out of the reach of children. |
|||
|
General Information about the safe and effective use of DUZALLO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DUZALLO for a condition for which it was not prescribed. Do not give DUZALLO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about DUZALLO that is written for health professionals. |
|||
|
What are the ingredients in DUZALLO? Active ingredients: lesinurad and allopurinol Inactive ingredients: crospovidone, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, iron oxide red, iron oxide yellow, titanium dioxide, and triacetin. DUZALLO 200/200 mg tablets are coated with Opadry orange and DUZALLO 200/300 mg tablets are coated with Opadry beige. Manufactured for: Ironwood Pharmaceuticals, Inc., Cambridge, MA 02142 By: AstraZeneca AB, SE-151 85 Sodertalje, Sweden. Ironwood and the three-leaf design are registered trademarks of Ironwood Pharmaceuticals, Inc. DUZALLO and ZURAMPIC are registered trademarks of the AstraZeneca group of companies. © AstraZeneca and Ironwood Pharmaceuticals, Inc. 2017 For more information, go to www.DUZALLO.com or call 1-844-374-4793. |
|||
| DUZALLO
lesinurad and allopurinol tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| DUZALLO
lesinurad and allopurinol tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Ironwood Pharmaceuticals, Inc. (054451401) |
Frequently asked questions
More about Duzallo (allopurinol / lesinurad)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antihyperuricemic agents



