Atopica for Cats (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
Cyclosporine oral solution, USP
100 mg/mL
VETERINARY USE ONLY
DIN: 02382490
Description
ATOPICA for Cats (cyclosporine oral solution, USP) is an oral formulation of cyclosporine that immediately forms a microemulsion in an aqueous environment. Cyclosporine, the active ingredient in ATOPICA for Cats, is a cyclic polypeptide immune modulating agent consisting of 11 amino acids. It is produced as a metabolite by the fungal species Beauveria nivea.
THERAPEUTIC CLASSIFICATION: Immunosuppressant - Calcineurin Inhibitor
INDICATION:
For the control of feline allergic dermatitis as manifested by excoriations (including face and neck), miliary dermatitis, eosinophilic plaques, and self-induced alopecia in cats at least 6 months of age.
Dosage and Administration
The initial dose of ATOPICA for Cats is 7 mg/kg/day as a single daily dose for 4 weeks. The clinical signs should improve during this 4 week period. Following this initial daily treatment period, the dose of ATOPICA for Cats may be tapered by decreasing the frequency of dosing to every other day and then twice weekly to maintain the desired therapeutic effect. Use the least frequent dosing schedule to control clinical signs. ATOPICA for Cats can be administered directly on a small amount of food or orally just after feeding. If a dose is missed, the next dose should be administered (without doubling) as soon as possible, but dosing should be no more frequent than once daily. If an unsatisfactory response is obtained within the first 4 weeks, the diagnosis and treatment should be re-evaluated.
Instructions For Dispensing Atopica For Cats
Unscrew the screw cap from the bottle, remove the rubber stopper and assemble the dispensing system as described below. Take out the required volume of the ATOPICA for Cats according to the weight of your cat by using the scale on the syringe and following the instructions given by your veterinarian. For the dosing process, carefully follow the handling/dispensing instructions as described below.
Preparing The Dispensing System
The dispensing system consists of 4 parts:

1. A bottle containing the medicine, with rubber stopper and a child-resistant screw cap to close the bottle after use.
2. A plastic adapter with dip tube that you will push into the neck of the bottle. The adapter must always remain in the bottle after first use.
3. An oral dosing syringe that fits into the plastic adapter to withdraw the prescribed dose of medicine from the bottle.
4. A plastic vial containing the plastic adapter and oral dosing syringe. Save the plastic vial to store the oral dosing syringe between each use.
Fitting the Plastic Adapter into the New Bottle of Medicine

● Remove the screw cap.
● Remove and dispose of the rubber stopper.
● Hold the open bottle upright on a table and push the plastic adapter firmly into the neck of the bottle as far as you can, then close the bottle with the screw cap.
Child-resistant closure of the bottle is achieved once the rubber stopper has been removed, the adapter pushed into the neck of the bottle and closed with the child-resistant screw cap.
Note: To dispense a dose, please follow all the instructions for Preparing a Dose of Medicine.
Preparing a Dose of Medicine

1. Push and turn the child-resistant cap to open the bottle.
Note: Always close the bottle with the child-resistant screw cap after use.
2. Check that the plunger of the syringe is pushed all the way down.
3. Keep the bottle upright and insert the syringe firmly into the plastic adapter.
4. Slowly pull the plunger up so that the syringe fills with the medicine.
5. Expel any large bubbles by pushing and pulling the plunger a few times. The presence of a few tiny bubbles is not important for dosing accuracy.
6. Withdraw the prescribed dose of medicine.
7. Remove the oral syringe by gently twisting it out of the plastic adapter.
You can now place the syringe over a small amount of food or introduce the syringe in the mouth of your pet and push the medicine out of the syringe.
Resealing Bottle to be Child-Resistant
1. After use, re-close the bottle with the given screw cap. To close the bottle you have to push the cap and screw. The product becomes child resistant once the child resistant-cap is screwed on the bottle with the presence of the plunger.
2. Push and turn the child-resistant cap to open the bottle.
Note: Always close the bottle with the child-resistant screw cap after use. Do not rinse or clean the oral dosing syringe between uses.
3. Store the oral dosing syringe in the plastic vial between each use.
CONTRA-INDICATIONS:
Do not use in cats with a history of malignant disorders or suspected malignancy. Do not use in cats infected with feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV). ATOPICA for Cats should not be used in cats with a hypersensitivity to cyclosporine.
CAUTIONS:
The safety and effectiveness of ATOPICA for Cats has not been established in cats less than 6 months of age or less than 1.4 kg body weight. ATOPICA for Cats is not for use in breeding cats, pregnant or lactating queens.
Cats should be tested and found negative for FeLV and FIV infections before treatment.
A complete clinical examination should be performed prior to treatment with ATOPICA for Cats.
The decision to treat with ATOPICA for Cats should be based on the cat’s clinical condition, response to prior treatments and in consideration of the benefit of treatment to the potential risks. Clinical signs of allergic dermatitis such as pruritus and skin inflammation are not specific for this disease. Other causes of dermatitis such as ectoparasitic infestations or food allergy should be evaluated and eliminated where possible. It is good practice to treat flea infestations before and during treatment of allergic dermatitis.
ATOPICA for Cats is a systemic immunosuppressant that may increase the susceptibility to infection and the development of neoplasia. As with any immunomodulation regimen, exacerbation of sub-clinical neoplastic conditions and infectious conditions may occur.
ATOPICA for Cats is not for use with other immunosuppressive agents. Of the 205 field study cats treated with ATOPICA for Cats, one died of the effusive form of feline infectious peritonitis and another cat was diagnosed with an indolent gastro-intestinal small cell lymphoma.
Persistent, progressive weight loss that resulted in hepatic lipidosis occurred in 2 of 205 cats treated with ATOPICA for Cats in field studies. Monitoring of body weight is recommended.
ATOPICA for Cats may cause increased concentrations of serum glucose, creatinine and urea nitrogen. ATOPICA for Cats should be used with caution in cases with diabetes mellitus or renal insufficiency.
ATOPICA for Cats should be used with caution with drugs that affect the P-450 enzyme system. Simultaneous administration of ATOPICA for Cats with drugs that suppress the P-450 enzyme system, such as azoles e.g. miconazole in ear drops, may lead to increased plasma levels of cyclosporine.
Cats that are seronegative for Toxoplasma gondii may be at risk of developing clinical toxoplasmosis if they become infected while under treatment, which can be fatal. In a controlled laboratory animal study, cats seronegative for T. gondii were administered cyclosporine and subsequently infected with T. gondii, resulting in increased susceptibility to infection and subsequent expression of toxoplasmosis. Cyclosporine was shown to not increase Toxoplasma oocyst shedding (see Safety). Potential exposure of seronegative cats to Toxoplasma should therefore be avoided (e.g. keep indoors, do not feed raw meat and prevent from hunting). In cases of clinical toxoplasmosis or other serious systemic illness, stop treatment with cyclosporine and initiate appropriate therapy.
Treatment with ATOPICA for Cats may result in decreased immune response to vaccination. Naïve cats may not develop protective titres during treatment (see Safety).
Warnings
Keep out of reach of children.
Wash hands after administration.
In case of accidental ingestion, seek medical advice immediately and provide the package insert or the label to the physician.
People with known hypersensitivity to cyclosporine should avoid contact with ATOPICA for Cats.
Adverse Reactions
The clinical safety of ATOPICA for Cats was assessed in a blinded, controlled 6-week field study followed by a 12 week open-labelled dose-tapering field study. In these two efficacy studies, 205 cats received treatment with ATOPICA for Cats for up to 126 days. Two cats died or were euthanized within 2 weeks following study exit (from the 12 week study). One cat was diagnosed with feline infectious peritonitis (FIP) and subsequently died following normal study exit and one cat with pre-existing anemia that worsened during the study was diagnosed with aplastic anemia and euthanized due to a poor prognosis for recovery.
The major reason for early withdrawal was lack of effectiveness. See table below for reason of withdrawal by study.
|
|
Number of Cases (Percent) |
||
|
6 Week Field Study |
12 Week Field Study |
||
|
Reason for Withdrawal |
ATOPICA for Cats n =144 |
Control Group n = 73 |
ATOPICA for Cats n = 191 |
|
Lack of Effectiveness |
12 (8.3%) |
29 (39.7%) |
15 (7.8%) |
|
Adverse Reaction |
2 (1.4%) |
1 (1.4%) |
12 (6.3%) |
|
Other (owner unable to medicate, owner non-compliance, loss to follow-up) |
5 (3.5%) |
4 (5.5%) |
9 (4.7%) |
Fourteen of the 205 (6.8%) cats which received cyclosporine in the two studies were withdrawn from the studies due to the occurrence of an adverse reaction. Adverse reactions in these 14 cats included weight loss, anorexia, vomiting, diarrhea, hypersalivation, lethargy, hepatic lipidosis and jaundice, upper respiratory tract signs, ocular discharge, cough, toxoplasmosis, lymphopenia, anemia, bacterial dermatitis, seizure, ataxia and gastrointestinal small cell lymphoma.
The most commonly reported adverse reaction was vomiting. In most cases, vomiting spontaneously resolved with continued dosing. Adverse reactions occurred most often on daily dosing compared to other dosing regimes.
|
Adverse Reaction* |
Number of Cases (Percent) N = 205 |
|
Vomiting/Retching/Regurgitation |
72 (35.1%) |
|
Weight Loss |
42 (20.5%) |
|
Diarrhea |
31 (15.1%) |
|
Anorexia/Decreased Appetite |
29 (14.1%) |
|
Lethargy/Malaise |
28 (13.6%) |
|
Hypersalivation |
23 (11.2%) |
|
Behavioural Disorder (hiding, hyperactivity, aggression) |
18 (8.8%) |
|
Ocular Discharge/Epiphora/Conjunctivitis |
14 (6.8%) |
|
Sneezing/Rhinitis |
11 (5.4%) |
|
Gingivitis/Gingival Hyperplasia |
9 (4.4%) |
|
Polydipsia |
6 (2.9%) |
*Cats may have experienced more than one type or occurrence of an event during the studies.
The following adverse reactions were reported in less than or equal to 2% of cats treated with ATOPICA for Cats in two field studies: bacterial dermatitis, hepatic lipidosis and jaundice, gastrointestinal small cell lymphoma, constipation, cough, toxoplasmosis, muscle wasting, muscle tremors, ataxia, convulsions, polyuria, urinary tract infection, inappropriate urination or defecation, seborrhoea, worsening otitis externa, papilloma, leukotrichia and excessive hair growth, anemia, lymphopenia, worsening monocytosis, worsening neutrophilia, hyperglobulinemia, increased serum creatinine and urea nitrogen and increased alanine transferase.
Of the 205 cats that received ATOPICA for Cats in the two field studies:
25 (12.2%) had Toxoplasma gondii titres go from negative to positive (20 of these developed IgM titres only)
9 (4.4%) had Toxoplasma gondii titres go from positive to negative
5 (2.4%) had Toxoplasma gondii titres go from negative to positive and return to negative while on ATOPICA for Cats
3 (1.4%) that began with positive Toxoplasma gondii titres had greater than 2-fold increases in IgG titres
One cat that developed a positive titre was diagnosed with clinical toxoplasmosis and subsequently recovered following discontinuation of ATOPICA for Cats and appropriate treatment.
One cat tested positive for Bartonella using the FeBart test while on treatment with ATOPICA for Cats.
To report suspected adverse reactions or for technical assistance, call Elanco Canada Limited Customer Service at 1-800-265-5475.
INFORMATION FOR OWNERS:
See end of package insert for information for cat owners.
Clinical Pharmacology
Cyclosporine is a calcineurin inhibitor which acts as an immunosuppressive agent that has been shown to work via suppression of T-helper and T-suppressor cells and inhibition of interleukin-2. ATOPICA for Cats is not a corticosteroid or antihistamine.
The average absolute bioavailability of cyclosporine A in cats following a single oral dose ranges from 23 to 29% depending on prandial state (fed versus fasted) and whether the drug is administered on food or given directly into the mouth. Following an intravenous dose of 2 mg/kg in a 24-hour fasted state, clearance (CL) of cyclosporine A in cats was 0.199 L/kg x h and half life (t1/2) was ~24 hours.
The bioavailability of ATOPICA for Cats is highly variable both within and between cats. A pharmacokinetic study showed no consistent difference in the mean extent of drug absorption when administered orally to fed or fasted cats or mixed in with food.
Blood levels of cyclosporine in field studies were highly variable, even among cats with similar clinical response, suggesting no general correlations can be made between cats with regard to blood cyclosporine levels and clinical response (effectiveness and safety). Nevertheless, individual differences in the relationship between drug exposure and clinical response may exist. Therefore, to minimize individual fluctuations in drug absorption, ATOPICA for Cats should be administered on a consistent schedule with regard to meals and time of day.
Cyclosporine is known to be extensively metabolized by cytochrome P-450 enzymes in the liver and prone to drug interactions (see Cautions section).
Efficacy Studies:
A masked, placebo-controlled, multi-centre field study was conducted at 24 sites from various geographic locations in the United States of America and Canada. In this study, 217 client owned cats presenting with clinical signs consistent with allergic dermatitis (miliary dermatitis, excoriations including the face or neck, self-induced alopecia and eosinophilic plaques) along with non-seasonal localized or generalized pruritus, were randomly assigned in a 2:1 ratio and received either ATOPICA for Cats or a control solution (the excipients of ATOPICA for Cats without the cyclosporine). Owners administered treatment in a small amount of food or directly in the cat’s mouth just after feeding once daily for up to 6 weeks. No additional therapy with antihistamines, corticosteroids or medicated shampoos was permitted.
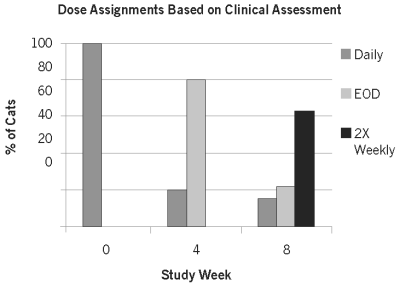
Efficacy was evaluated in 181 cats. Cats in the ATOPICA for Cats treatment group had a 65.1% reduction in mean total lesion score compared to cats in the control group which had a 9.2% reduction in mean total lesion score. The percent of cats identified by Owner as successfully treated was 78.6% in the ATOPICA for Cats treatment group compared to 26.2% in the control group. Compared to the control group, the ATOPICA for Cats group had improved mean ratings for Investigator assessment of overall clinical improvement, Owner and Investigator assessment of pruritus and number of body regions with lesions. After drop-out from or completion of the blinded 6 week field study, 191 cats were enrolled into a multi-centre, open-labelled field trial to evaluate dose tapering of ATOPICA for Cats according to the clinical response. The graph below shows the dose assignments for each 4-week dosing period. At study entry, all cats were assigned daily doses. At Week 4, cats were assigned daily or every other day (EOD) dosing, based on clinical improvement. At Week 8, cats were assigned daily, EOD or twice weekly (2x Weekly) dosing for the final month of the study. Cats with poor responses exited the study at Weeks 4 and 8. At study exit at Week 12, 63%, 22% and 15% of the remaining 97 evaluable cats were on twice weekly, EOD and daily dosing regimens, respectively.
Blood levels of cyclosporine drawn during these studies were highly variable, even among cats with similar clinical response. A correlation between blood cyclosporine concentrations and clinical efficacy has not been possible to establish due to the high variability.
Safety Studies:
In a 6 month safety study, 40 (20 male and 20 female), 6-month old cats were randomized into 5 treatment groups and administered 0, 8, 16, 24 or 40 mg/kg/day ATOPICA for Cats (0, 1, 2, 3 or 5X the maximum therapeutic dose). Intermittent interventricular conduction disturbances were noted in one 5x cat who had a right bundle branch block and one 3x cat who had electrical alternans following 6 months of dosing. A 5x cat was euthanized after 2 weeks of treatment following a rapid decline. The cat was recumbent, anorexic, dehydrated and losing weight. A post-mortem examination showed a healing rib fracture and bone marrow hypocellularity from multiple cell lines. Hematology done prior to euthanasia did not reveal abnormalities indicative of bone marrow hypocellularity. A 5x female developed abdominal fibroadematous nodules during the study. Lymphoma of the kidneys and a mesenteric lymph node were present on the necropsy in one 5x male which is likely related to the immunosuppressive effects of cyclosporine treatment. Activated partial thromboplastin (APTT) time was prolonged in treated cats when compared to control cats. There was a dose-related increase in the frequency and number of male cats with observations of ‘soft feces’ during the study. This observation was considered treatment-related.
Pharmacokinetic evaluations revealed non-linearity in important pharmacokinetic parameters (AUC, Cmax) with increasing dose. There was no evidence of bioaccumulation after steady state was achieved at the 1X dose level.
A safety study was conducted to evaluate the effect of ATOPICA for Cats on the development of vaccine titres following vaccination. Thirty-two cats (16 males and 16 females) were randomized into 2 treatment groups. Group 1 was the control group and was sham dosed. Group 2 cats were administered ATOPICA for Cats at a dose of 24 mg/kg (3x the maximum therapeutic dose) orally once daily for 56 days. All cats were approximately 7-months old at the start of the study and previously vaccinated against feline calici virus (FCV), feline panleukopenia virus (FPV), FeLV, feline herpes virus-1 (FHV-1) and rabies. The last pre-enrollment vaccines (FVRCP, rabies) were administered approximately 16 weeks prior to the first administration of ATOPICA for Cats. Cats were naïve to the feline immunodeficiency virus (FIV) vaccine, which was administered after 28 days on cyclosporine. After booster vaccinations on day 28, titres for FCV, FPV, FeLV, FHV-1 and rabies were decreased in cyclosporine treated cats compared to control cats, but these titres remained adequate in both treatment groups. In contrast, cats on high dose cyclosporine failed to develop titres to the novel vaccine (FIV). An increase incidence and frequency of diarrhea, and salivation were noted in group 2 cats. One female cat treated with cyclosporine was observed to be in estrus during the study compared to 5 of the female control cats. One cat treated with cyclosporine was noted as having a slow or absent startle reflex, displayed ataxia, had small lymph nodes, thin body condition, and gas filled loops of intestines. Lymphocyte counts were lower in treated cats compared to control. APTT was prolonged in treated cats when compared to controls. Cholesterol, glucose, total protein, blood urea nitrogen and creatinine values were increased in cyclosporine treated cats with values just above the normal range. Glucosuria was noted in 3 cyclosporine treated cats that were also hyperglycemic.
A safety study was conducted to evaluate the effects of ATOPICA for Cats on the clinical course of Toxoplasmosis gondii. Thirty cats (15 male and 15 female) ranging in age from 1 to 2 years were randomized into 3 treatment groups. Group 1 served as the control group and were administered a placebo for 126 days. Group 2 cats were administered a placebo for 84 days followed by treatment with ATOPICA for Cats for 42 days. Group 3 cats were treated with ATOPICA for Cats for 126 days. ATOPICA for Cats was administered at a target dose of 7.5 mg/kg orally once daily. All cats were infected with T. gondii cysts on day 42. One cat was found dead and another was euthanized (both Group 3) within 6 weeks following infection due to complications related to toxoplasmosis. Clinical signs typical of T. gondii infection, including bloody feces, lethargy, vomiting/regurgitation, were also seen in most of the remaining cats, but resolved within 6 weeks following infection. Decreases in body weight and food consumption were seen in some cats from each group, but these changes were reversible as the cats recovered from clinical toxoplasmosis. APTT was prolonged in Group 2 and 3 cats receiving cyclosporine when compared to Group 1 cats. Cholesterol, glucose and total protein/globulin values were increased in cyclosporine treated cats. Ocular changes consistent with toxoplasmosis were seen in one to two cats in each group. The oocyst shedding period and number of oocysts shed were increased in Group 1 and Group 2 cats compared to Group 3 cats. All inoculated cats developed T. gondii IgG antibodies; IgM antibodies were detected in only 3 cats. Post mortem examinations revealed mild to moderate inflammation in the central nervous system (CNS) and pulmonary tissues, with the highest incidence and severity generally following this trend: Group 3 > Group 2 > Group 1. Lesions were consistent with T. gondii infection and were often more prevalent in males than females. T. gondii organisms were only detected histopathologically in tissues of the 2 Group 3 cats that died of toxoplasmosis.examined histopathologically.
Storage
ATOPICA for Cats should be stored and dispensed in the original container at controlled room temperature between 15-25°C.
Once opened, use contents within 7 weeks for the 5 mL container and 11 weeks for the 17 mL container.
PRODUCT PRESENTATION:
ATOPICA for Cats is a clear yellow to brownish solution packaged in a multi-dose amber glass vial closed with a rubber stopper and sealed with a child-resistant screw cap. Each carton of product contains one vial and a dispenser set (consisting of a dip tube, plastic vial and a 1 mL syringe).
ATOPICA for Cats is available in 5 mL and 17 mL sizes.
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
Date: May 2022
Atopica, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
01Jun2022
CPN: 1231106.3
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
