Virasal Prescribing Information
Package insert / product label
Generic name: salicylic acid
Dosage form: topical solution
Drug class: Topical keratolytics
Medically reviewed by Drugs.com. Last updated on Mar 1, 2024.
On This Page
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle - Package Insert Text:
DESCRIPTION
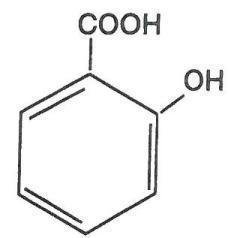
Virasal is a topical preparation containing 27.5% salicylic acid in a proprietary film-forming vehicle that comprises isopropyl alcohol, butyl acetate, polyvinyl butyral, isopropyl metacresol, trimethyl pentanyl diisobutyrate, phenic acid and acrylates copolymer. The pharmacologic activity of Virasal is generally attributed to the keratolytic activity of salicylic acid which is incorporated into a polyacrylic, film-forming virucidal vehicle designed to cover the wart without the need for a bandage. The structural formula of salicylic acid is:
CLINICAL PHARMACOLOGY
Although the exact mode of action for salicylic acid in the treatment of warts is unknown, its activity appears to be associated with its keratolytic action, which results in mechanical removal of epidermal cells infected with wart viruses.
The virucidal complex incorporated into Virasal’s proprietary vehicle is designed to help reduce risk of reinfection at the wart site, as well as prevent viral contamination of the product under normal usage.
INDICATIONS AND USAGE
Virasal is indicated for the topical treatment and removal of common warts and plantar warts.
CONTRAINDICATIONS
Patients with diabetes or impaired blood circulation should not use Virasal. Virasal also should not be used on moles, birthmarks, and unusual warts with hair growing from them, or warts on the face.
PRECAUTIONS
Virasal is for external use only. Do not permit Virasal to contact eyes or mucous membranes. If contact with eyes or mucous membranes occurs, immediately flush with water for 15 minutes. Virasal should not be allowed to contact normal skin surrounding wart, since localized irritation may occur. Treatment should be discontinued if excessive irritation occurs. Virasal is flammable. Keep away from fire or flame. Keep bottle tightly capped when not in use.
ADVERSE REACTIONS
A localized irritant reaction may occur if Virasal is applied to the normal skin surrounding the wart. Any irritation may normally be controlled by temporarily discontinuing use and by applying the medication only to the wart site when treatment is resumed.
DOSAGE AND ADMINISTRATION
Prior to applying Virasal, soak wart in warm water for five minutes. Remove any loosened tissue by gently rubbing with a brush, wash cloth, or emery board. Dry wart site thoroughly. Using the brush applicator supplied, apply Virasal twice to entire wart surface, allowing the first application to dry before applying the second. Continue treatment once or twice a day as directed by physician. Be careful not to apply to surrounding skin.
Clinically visible improvement will normally occur during the first or second week of therapy. Maximum resolution may be expected after four to six weeks of Virasal use.
Related/similar drugs
salicylic acid topical, cantharidin topical, silver nitrate topical, Compound W, Wart Remover, Duofilm
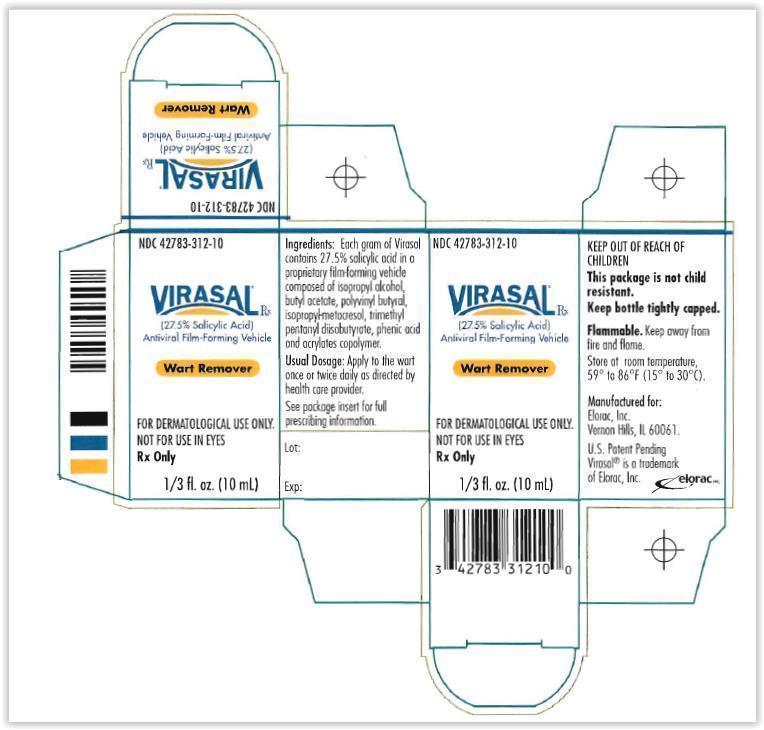
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle - Container Label Principal Display Panel Text:
NDC 42783-312-10
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle
Wart Remover
FOR TOPICAL USE ONLY
NOT FOR USE IN THE EYES
Rx only
1/3 fl. oz (10 mL)

| VIRASAL
salicylic acid solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Elorac, Inc. (832590009) |
| Registrant - Elorac, Inc. (832590009) |
Frequently asked questions
More about Virasal (salicylic acid topical)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- Drug class: topical keratolytics
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Salvax, Keralyt Shampoo, KeralytGel, Rayasal, ... +5 more