Sodium Fluoride Drops: Package Insert / Prescribing Info
Package insert / product label
Dosage form: oral solution/drops
Drug class: Minerals and electrolytes
Medically reviewed by Drugs.com. Last updated on Dec 13, 2023.
On This Page
Sodium Fluoride Drops Description
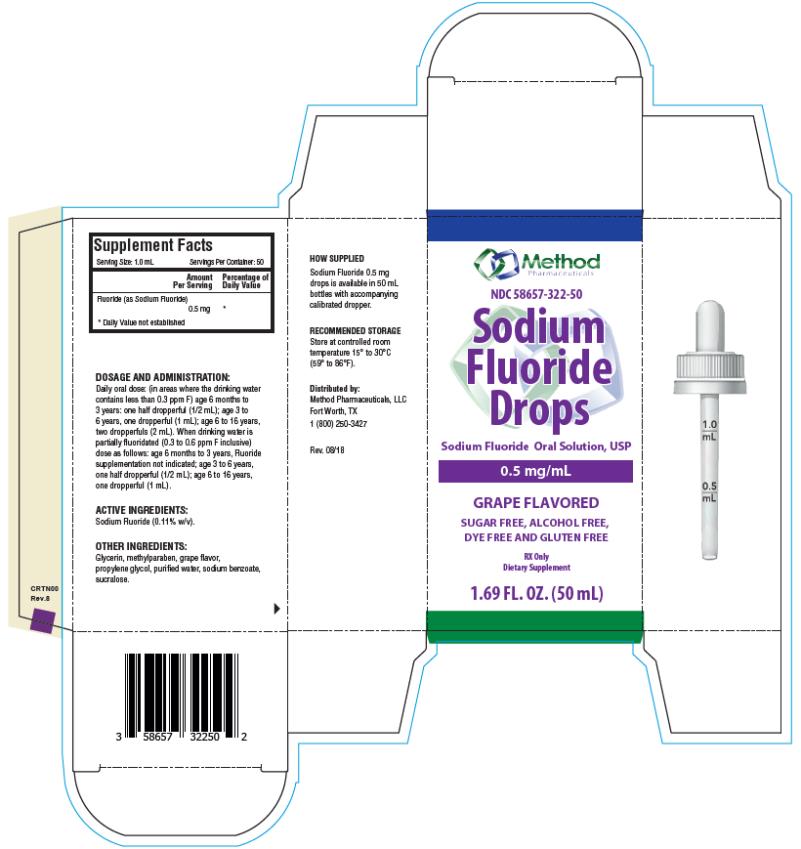
Each mL of Sodium Fluoride Drops contains 0.5 mg Fluoride ion (F) from 1.1 mg Sodium Fluoride (NaF). For use as a dental caries preventive in pediatric patients. Sugar Free, Alcohol Free, Dye Free and Gluten Free.
Supplement Facts
Serving Size: 1 mL
Servings Per Container: 50
Amount per serving % Daily Value
Fluoride (as Sodium Fluoride) 0.5 mg **
** Daily Value not established.
Active Ingredients: Sodium Fluoride (0.11% w/v).
Other Ingredients: Glycerin, methylparaben, grape flavor, propylene glycol, purified water, sodium benzoate, sucralose.
| FLUORIDE SUPPLEMENT DOSAGE SCHEDULES | |||
| AGE | Fluoride Ion Level in Drinking Water (ppm)* | ||
| < 0.3 ppm | 0.3 - 0.6 ppm | > 0.6 ppm | |
| Birth to 6 months | None | None | None |
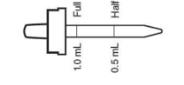
| 6 months to 3 years | Half dropperful 0.25 mg F (1/2 mL) | None | None |
| 3 to 6 years | One dropperful 0.5 mg F (1 mL)† | Half dropperful 0.25 mg F (1/2 mL) | None |
| 6 to 16 years | Two dropperfuls 1 mg F (2 mL) | One dropperful 0.5 mg F (1 mL) | None |
* 1.0 ppm = 1 mg/Liter
† 1.1 mg Sodium Fluoride contains 0.5 mg Fluoride ion
Fluoride Supplement Dose Schedule approved by the American Dental Association, American Academy of Pediatrics and American Academy of Pediatric Dentistry.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Sodium Fluoride Drops - Clinical Pharmacology
Sodium Fluoride acts systemically (before tooth eruption) and topically (post eruption) by increasing tooth resistance to acid dissolution, by promoting remineralization and by inhibiting the cariogenic microbial process.
Indications and Usage for Sodium Fluoride Drops
As a supplemental source of Fluoride. It has been established that ingestion of fluoridated drinking water (1 ppm F) during the period of tooth development results in significant decrease in the incidence of dental caries.1 Sodium Fluoride Drops were developed to provide systemic Fluoride for use as a supplement
in pediatric patients from 6 months to age 3 and older, living in areas where the drinking water Fluoride level does not exceed 0.6 ppm F.
Contraindications
Do not use in areas where drinking water exceeds 0.6 ppm F. Do not administer to pediatric patients less than 6 months old.
Warnings
Prolonged daily ingestion of quantities greater than the recommended amount may result in various degrees of dental fluorosis in pediatric patients under age 6 years, especially if the water fluoridation exceeds 0.6 ppm. Read directions carefully before using. Keep out of the reach of infants and children.
Precautions
See " Overdosage" section. Incompatibility of Fluoride with dairy foods has been reported due to formation of Calcium Fluoride which is poorly absorbed. Not for use in the eyes.
Adverse Reactions/Side Effects
Allergic rash and other idiosyncrasies have been rarely reported.
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or Method Pharmaceuticals, LLC at 877-250-3427.
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Overdosage
Prolonged daily ingestion of excessive Fluoride may result in varying degrees of dental fluorosis. The total amount of Sodium Fluoride in a bottle of 50 mL (0.5 mg/mL) Sodium Fluoride Drops (25 mg F) conforms with the recommendations of the American Dental Association for the maximum to be dispensed at one time for safety purposes. If overdose is suspected, call 1-800-222-1222 (American Association of Poison Control Centers), your local poison control center (www.aapcc.org), or emergency room immediately for treatment recommendations.
Dosages and Administration
Daily oral dose: (in areas where the drinking water contains less than 0.3 ppm F) age 6 months to 3 years: one half dropperful (1/2 mL); age 3 to 6 years, one dropperful (1 mL); age 6 to 16 years, two dropperfuls (2 mL). When drinking water is partially fluoridated (0.3 to 0.6 ppm F inclusive) dose as follows: age 6 months to 3 years, Fluoride supplementation not indicated; age 3 to 6 years, one half dropperful (1/2 mL); age 6 to 16 years, one dropperful (1 mL).
| SODIUM FLUORIDE
sodium fluoride solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Method Pharmaceuticals, LLC (060216698) |
More about fluoride
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Drug class: minerals and electrolytes
- En español
Patient resources
Professional resources
Other brands
Fluor-A-Day, Fluorabon Drops, Flura-Drops