Sodium Chloride Inhalation Solution: Package Insert / Prescribing Info
Package insert / product label
Dosage form: inhalation solution
Drug classes: Minerals and electrolytes, Miscellaneous respiratory agents
Medically reviewed by Drugs.com. Last updated on Feb 28, 2024.
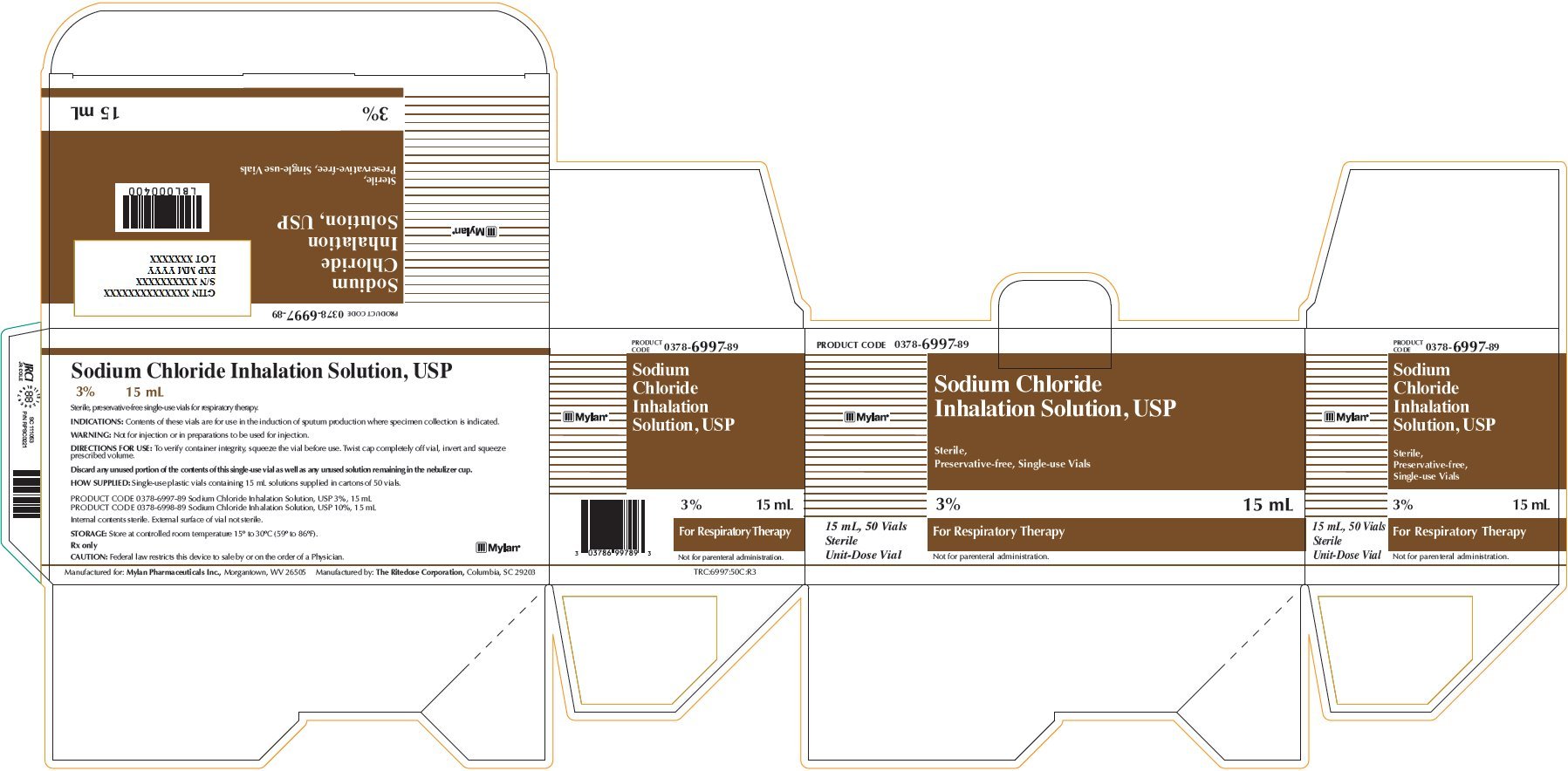
Sodium Chloride Inhalation Solution 3% 15 mL
PRODUCT CODE 0378-6997-89
Sodium Chloride
Inhalation Solution, USP
Sterile,
Preservative-free, Single-use Vials
3% 15 mL
For Respiratory Therapy
Not for parenteral administration.
15 mL, 50 Vials
Sterile
Unit-Dose Vial
Sodium Chloride Inhalation Solution, USP
3% 15 mL
Sterile, preservative-free single-use vials for respiratory therapy.
INDICATIONS: Contents of these vials are for use in the induction of sputum production where specimen collection is indicated.
WARNING: Not for injection or in preparations to be used for injection.
DIRECTIONS FOR USE: To verify container integrity, squeeze the vial before use. Twist cap completely off vial, invert and squeeze prescribed volume.
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
HOW SUPPLIED: Single-use plastic vials containing 15 mL solutions supplied in cartons of 50 vials.
PRODUCT CODE 0378-6997-89 Sodium Chloride Inhalation Solution, USP 3%, 15 mL
PRODUCT CODE 0378-6998-89 Sodium Chloride Inhalation Solution, USP 10%, 15 mL
Internal contents sterile. External surface of vial not sterile.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
CAUTION: Federal law restricts this device to sale by or on the order of a Physician.
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 Manufactured by: The Ritedose Corporation, Columbia, SC 26203
TRC:6997:50C:R3
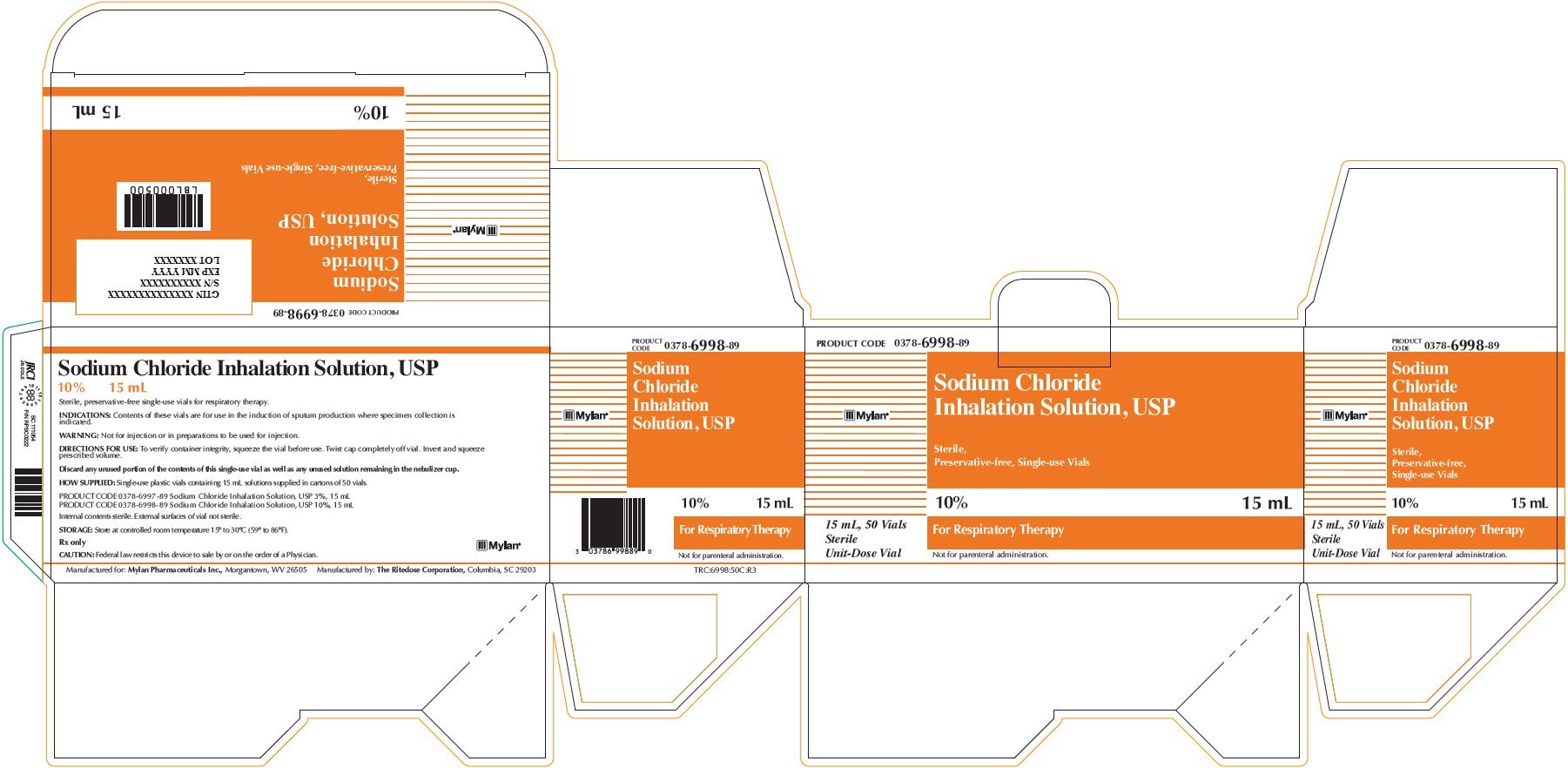
Sodium Chloride Inhalation Solution 10% 15 mL
PRODUCT CODE 0378-6998-89
Sodium Chloride
Inhalation Solution, USP
Sterile,
Preservative-free, Single-use Vials
10% 15 mL
For Respiratory Therapy
Not for parenteral administration.
15 mL, 50 Vials
Sterile
Unit-Dose Vial
Sodium Chloride Inhalation Solution, USP
10% 15 mL
Sterile, preservative-free single-use vials for respiratory therapy.
INDICATIONS: Contents of these vials are for use in the induction of sputum production where specimen collection is indicated.
WARNING: Not for injection or in preparations to be used for injection.
DIRECTIONS FOR USE: To verify container integrity, squeeze the vial before use. Twist cap completely off vial. Invert and squeeze prescribed volume.
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
HOW SUPPLIED: Single-use plastic vials containing 15 mL solutions supplied in cartons of 50 vials.
PRODUCT CODE 0378-6997-89 Sodium Chloride Inhalation Solution, USP 3%, 15 mL
PRODUCT CODE 0378-6998-89 Sodium Chloride Inhalation Solution, USP 10%, 15 mL
Internal contents sterile. External surface of vial not sterile.
STORAGE: Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
CAUTION: Federal law restricts this device to sale by or on the order of a Physician.
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 Manufactured by: The Ritedose Corporation, Columbia, SC 26203
TRC:6998:50C:R3
| SODIUM CHLORIDE
nebulizer (direct patient interface) |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
nebulizer (direct patient interface) |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Pharmaceuticals Inc. (059295980) |
Frequently asked questions
More about sodium chloride
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (3)
- Drug images
- Latest FDA alerts (19)
- Side effects
- Drug class: minerals and electrolytes
Patient resources
Professional resources
- Sodium Chloride monograph
- Sodium Chloride 20% Injection (AHFS Monograph)
- Bacteriostatic Sodium Chloride (FDA)
- Sodium Chloride 0.45% Injection (FDA)
- Sodium Chloride Injection (FDA)