Rotarix Prescribing Information
Package insert / product label
Generic name: rotavirus vaccine, live
Dosage form: oral applicator
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Jan 31, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ROTARIX (Rotavirus Vaccine, Live, Oral)
Suspension, for oral use
Initial U.S. Approval: 2008
Indications and Usage for Rotarix
ROTARIX is a vaccine indicated for the prevention of rotavirus gastroenteritis caused by G1 and non-G1 types (G3, G4, and G9). ROTARIX is approved for use in infants 6 weeks and up to 24 weeks of age. (1)
Rotarix Dosage and Administration
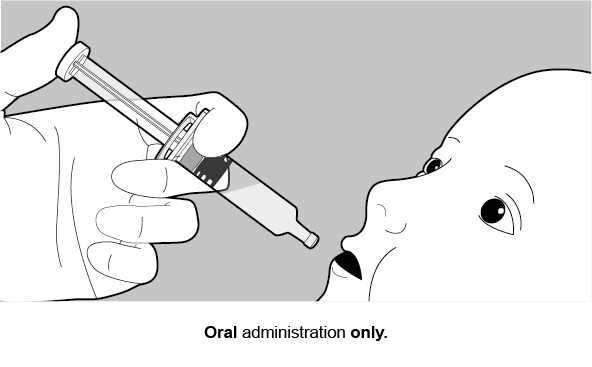
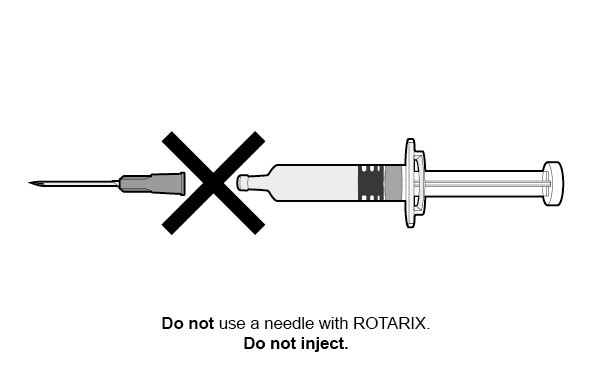
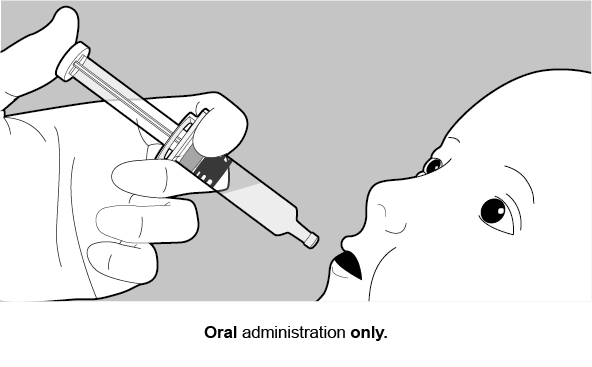
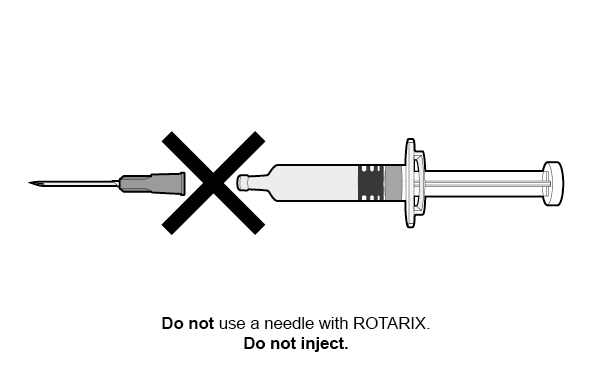
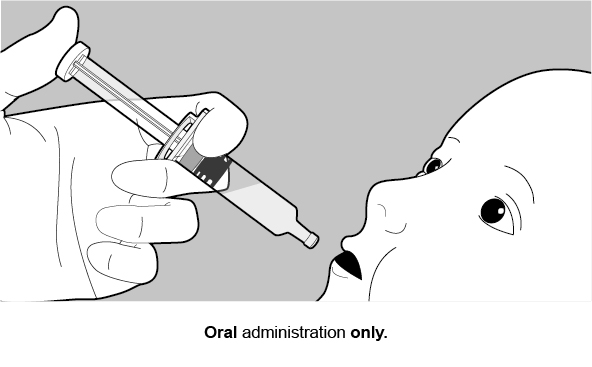
FOR ORAL USE ONLY.
ROTARIX is supplied as either:
- •
- Vial and oral dosing applicator presentation: The vial contains the lyophilized vaccine component and the oral dosing applicator contains the diluent. The contents of the vial must be reconstituted with the diluent to form ROTARIX prior to administration (2.1), or
- •
- Oral dosing applicator only presentation: The oral dosing applicator contains ROTARIX and does NOT require reconstitution or dilution before use. (2.1)
Schedule
Dosage Forms and Strengths
Contraindications
- •
- A demonstrated history of hypersensitivity to the vaccine or any component of the vaccine. (4.1, 11)
- •
- History of uncorrected congenital malformation of the gastrointestinal tract that would predispose the infant to intussusception. (4.2)
- •
- History of intussusception. (4.3)
- •
- History of Severe Combined Immunodeficiency Disease (SCID). (4.4, 6.2)
Warnings and Precautions
- •
- The tip caps of the prefilled oral dosing applicators contain natural rubber latex which may cause allergic reactions. (5.1)
- •
- Administration of ROTARIX in infants suffering from acute diarrhea or vomiting should be delayed. Safety and effectiveness of ROTARIX in infants with chronic gastrointestinal disorders have not been evaluated. (5.2)
- •
- Safety and effectiveness of ROTARIX in infants with known primary or secondary immunodeficiencies have not been established. (5.3)
- •
- In a postmarketing study, cases of intussusception were observed in temporal association within 31 days following the first dose of ROTARIX, with a clustering of cases in the first 7 days. (5.5, 6.2)
Adverse Reactions/Side Effects
Common (≥5%) solicited adverse reactions included fussiness/irritability, cough/runny nose, fever, loss of appetite, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2024
Full Prescribing Information
1. Indications and Usage for Rotarix
ROTARIX is indicated for the prevention of rotavirus gastroenteritis caused by G1 and non-G1 types (G3, G4, and G9) when administered as a 2-dose series [see Clinical Studies (14.3)]. ROTARIX is approved for use in infants 6 weeks and up to 24 weeks of age.
2. Rotarix Dosage and Administration
For oral use only.
2.1 ROTARIX Presentations
ROTARIX is supplied in two presentations, a vial and oral dosing applicator presentation and an oral dosing applicator only presentation.
Vial and Oral Dosing Applicator Presentation
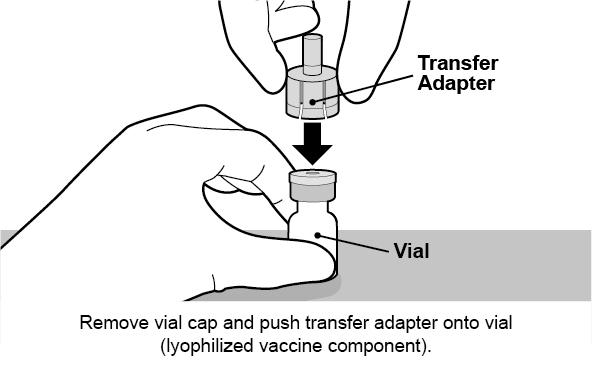
The vial contains the lyophilized vaccine component, and the oral dosing applicator contains the diluent. The contents of the vial must be reconstituted with the diluent to form ROTARIX prior to administration.
Oral Dosing Applicator Only Presentation
The oral dosing applicator only presentation contains ROTARIX and does NOT require reconstitution or dilution before use.
2.2 Preparation and Administration
Vial and Oral Dosing Applicator Presentation
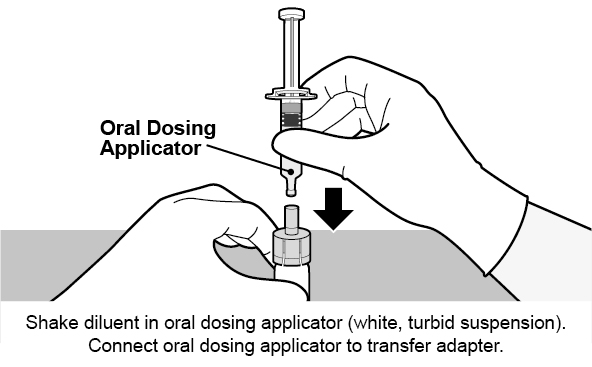
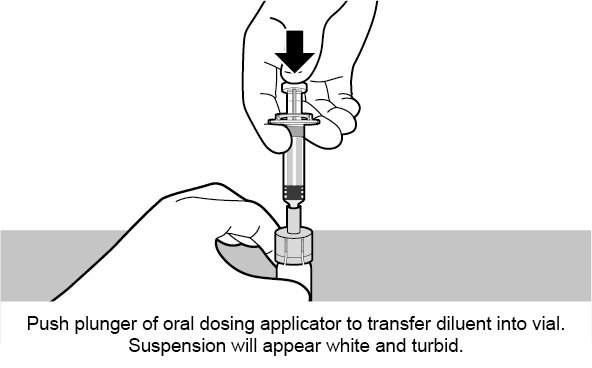
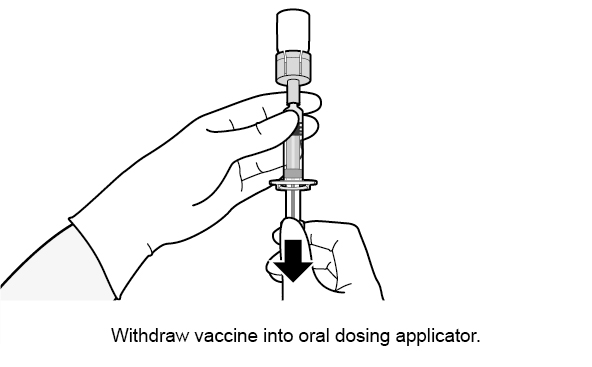
Use accompanying diluent to reconstitute the lyophilized vaccine component to form ROTARIX. After reconstitution, each dose of 1 mL is administered orally.
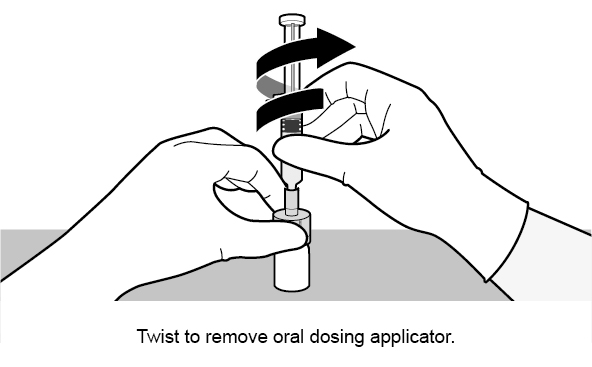
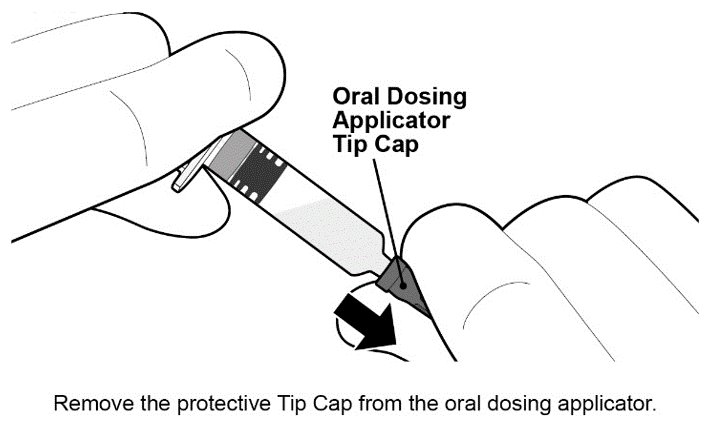
See Figure 1 for preparation and administration steps.
Figure 1. Preparation and Administration Steps of Vial and Oral Dosing Applicator Presentation
Oral Dosing Applicator Only Presentation
The ROTARIX oral dosing applicator only presentation does NOT require reconstitution or dilution before use. Each dose of 1.5 mL is administered orally.
See Figure 2 for preparation and administration steps.
Figure 2. Preparation and Administration Steps of Oral Dosing Applicator Only Presentation
2.3 Dosing and Schedule
The vaccination series consists of two doses administered orally. The first dose should be administered to infants beginning at 6 weeks of age. There should be an interval of at least 4 weeks between the first and second dose. The 2-dose series should be completed by 24 weeks of age.
Safety and effectiveness have not been evaluated if ROTARIX were administered for the first dose and another rotavirus vaccine were administered for the second dose or vice versa.
In the event that the infant spits out or regurgitates most of the vaccine dose, a single replacement dose may be considered at the same vaccination visit.
2.4 Infant Feeding
Breastfeeding was permitted in clinical studies. There was no evidence to suggest that breastfeeding reduced the protection against rotavirus gastroenteritis afforded by ROTARIX. There are no restrictions on the infant’s liquid consumption, including breast milk, either before or after vaccination with ROTARIX.
3. Dosage Forms and Strengths
Suspension for Oral Use
- •
- Vial and oral dosing applicator presentation: after reconstitution a single dose is 1 mL.
- •
- Oral dosing applicator only presentation: a single dose is 1.5 mL.
4. Contraindications
4.1 Hypersensitivity
A demonstrated history of hypersensitivity to any component of the vaccine.
Infants who develop symptoms suggestive of hypersensitivity after receiving a dose of ROTARIX should not receive further doses of ROTARIX.
4.2 Gastrointestinal Tract Congenital Malformation
Infants with a history of uncorrected congenital malformation of the gastrointestinal tract (such as Meckel’s diverticulum) that would predispose the infant for intussusception should not receive ROTARIX.
4.3 History of Intussusception
Infants with a history of intussusception should not receive ROTARIX [see Warnings and Precautions (5.5)]. In postmarketing experience, intussusception resulting in death following a second dose has been reported following a history of intussusception after the first dose [see Adverse Reactions (6.2)].
4.4 Severe Combined Immunodeficiency Disease
Infants with Severe Combined Immunodeficiency Disease (SCID) should not receive ROTARIX. Postmarketing reports of gastroenteritis, including severe diarrhea and prolonged shedding of vaccine virus, have been reported in infants who were administered live, oral rotavirus vaccines and later identified as having SCID [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Latex
The tip caps of the prefilled oral dosing applicators contain natural rubber latex which may cause allergic reactions.
5.2 Gastrointestinal Disorders
Administration of ROTARIX should be delayed in infants suffering from acute diarrhea or vomiting.
Safety and effectiveness of ROTARIX in infants with chronic gastrointestinal disorders have not been evaluated. [See Contraindications (4.2).]
5.3 Altered Immunocompetence
Safety and effectiveness of ROTARIX in infants with known primary or secondary immunodeficiencies, including infants with human immunodeficiency virus (HIV), infants on immunosuppressive therapy, or infants with malignant neoplasms affecting the bone marrow or lymphatic system have not been established.
5.4 Shedding and Transmission
Rotavirus shedding in stool occurs after vaccination with peak excretion occurring around Day 7 after Dose 1.
One clinical trial demonstrated that vaccinees transmit vaccine virus to healthy seronegative contacts [see Clinical Pharmacology (12.2)].
The potential for transmission of vaccine virus following vaccination should be weighed against the possibility of acquiring and transmitting natural rotavirus. Caution is advised when considering whether to administer ROTARIX to individuals with immunodeficient close contacts, such as individuals with malignancies, primary immunodeficiency or receiving immunosuppressive therapy.
5.5 Intussusception
Following administration of a previously licensed oral live rhesus rotavirus-based vaccine, an increased risk of intussusception was observed.1 The risk of intussusception with ROTARIX was evaluated in a pre-licensure randomized, placebo-controlled safety study (including 63,225 infants) conducted in Latin America and Finland. No increased risk of intussusception was observed in this clinical trial following administration of ROTARIX when compared with placebo. [See Adverse Reactions (6.1).]
In a postmarketing, observational study conducted in Mexico, cases of intussusception were observed in temporal association within 31 days following the first dose of ROTARIX, with a clustering of cases in the first 7 days. [See Adverse Reactions (6.2).]
Other postmarketing observational studies conducted in Brazil and Australia also suggest an increased risk of intussusception within the first 7 days following the second dose of ROTARIX.2,3[See Adverse Reactions (6.2).]
In worldwide passive postmarketing surveillance, cases of intussusception have been reported in temporal association with ROTARIX [see Adverse Reactions (6.2)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
There are two formulations of ROTARIX: a reconstituted lyophilized formulation (supplied in a vial and oral dosing applicator presentation) and a liquid formulation (supplied in an oral dosing applicator only presentation) [see Description (11)]. Safety data accrued with each formulation is relevant to the other because each contains the same live, attenuated rotavirus strain and is manufactured using a similar process.
Common (≥5%) solicited adverse reactions included fussiness/irritability, cough/runny nose, fever, loss of appetite, and vomiting.
Solicited adverse reactions, unsolicited adverse events, serious adverse events (SAEs), and cases of intussusception were collected in 7 clinical studies (Studies 1 to 7; NCT00729001, NCT00385320, NCT00429481, NCT00757770, NCT00140686, NCT00169455, NCT00137930). Cases of intussusception and SAEs were collected in an additional large safety study (Study 8; NCT00140673) that compared ROTARIX (reconstituted lyophilized formulation) to placebo. Solicited adverse reactions, unsolicited adverse events, and SAEs were collected in 3 clinical studies (Studies 9 to 11; NCT02914184, NCT03207750, NCT03954743) that compared the two ROTARIX formulations.
Clinical Trials Experience with ROTARIX (Reconstituted Lyophilized Formulation)
Studies 1 to 8 evaluated a total of 71,209 infants who received ROTARIX (n = 36,755) or placebo (n = 34,454). The racial distribution for these studies was as follows: Hispanic 73.4%, White 16.2%, Black 1.0%, and other 9.4%; 51% were male.
Solicited Adverse Reactions: In 7 clinical studies (Studies 1 to 7), detailed safety information was collected by parents/guardians for 8 consecutive days following vaccination with ROTARIX (i.e., day of vaccination and the next 7 days). A diary card was completed to record fussiness/irritability, cough/runny nose, the infant’s temperature, loss of appetite, vomiting, or diarrhea on a daily basis during the first week following each dose of ROTARIX or placebo. Adverse reactions among recipients of ROTARIX and placebo occurred at similar rates (Table 1).
| Total vaccinated cohort = All vaccinated infants for whom safety data were available. n = Number of infants for whom at least one symptom sheet was completed. a Defined as crying more than usual. b Data not collected in 1 of 7 studies; Dose 1: ROTARIX n = 2,583; placebo n = 1,897; Dose 2: ROTARIX n = 2,522; placebo n = 1,863. c Defined as temperature ≥100.4°F (≥38.0°C) rectally or ≥99.5°F (≥37.5°C) orally. d Defined as eating less than usual. |
||||
|
Adverse Reaction |
Dose 1 |
Dose 2 |
||
|
ROTARIX |
Placebo |
ROTARIX |
Placebo |
|
|
n = 3,284 |
n = 2,013 |
n = 3,201 |
n = 1,973 |
|
|
% |
% |
% |
% |
|
|
Fussiness/irritabilitya |
52 |
52 |
42 |
42 |
|
Cough/runny noseb |
28 |
30 |
31 |
33 |
|
Feverc |
25 |
33 |
28 |
34 |
|
Loss of appetited |
25 |
25 |
21 |
21 |
|
Vomiting |
13 |
11 |
8 |
8 |
|
Diarrhea |
4 |
3 |
3 |
3 |
Unsolicited Adverse Reactions: Infants were monitored for unsolicited serious and non-SAEs that occurred in the 31-day period following vaccination in 7 clinical studies (Studies 1 to 7). The following adverse reactions occurred at a statistically higher incidence (95% Confidence Interval [CI] of Relative Risk [RR] excluding 1) among recipients of ROTARIX (n = 5,082) as compared with placebo recipients (n = 2,902): irritability (ROTARIX 11.4%, placebo 8.7%) and flatulence (ROTARIX 2.2%, placebo 1.3%).
Serious Adverse Reactions: Infants were monitored for SAEs that occurred in the 31-day period following vaccination in 8 clinical studies (Studies 1 to 8). Serious adverse reactions occurred in 1.7% of recipients of ROTARIX (n = 36,755) as compared with 1.9% of placebo recipients (n = 34,454). Among placebo recipients, diarrhea (placebo 0.07%, ROTARIX 0.02%), dehydration (placebo 0.06%, ROTARIX 0.02%), and gastroenteritis (placebo 0.3%, ROTARIX 0.2%) occurred at a statistically higher incidence (95% CI of RR excluding 1) as compared with recipients of ROTARIX.
Deaths: During the entire course of 8 clinical studies (Studies 1 to 8), there were 68 (0.19%) deaths following administration of ROTARIX (n = 36,755) and 50 (0.15%) deaths following placebo administration (n = 34,454). The most commonly reported cause of death following vaccination was pneumonia, which was observed in 19 (0.05%) recipients of ROTARIX and 10 (0.03%) placebo recipients (RR: 1.74, 95% CI: 0.76, 4.23).
Intussusception: In a controlled safety study (Study 8) conducted in Latin America and Finland, the risk of intussusception was evaluated in 63,225 infants (31,673 received ROTARIX and 31,552 received placebo). Infants were monitored by active surveillance including independent, complementary methods (prospective hospital surveillance and parent reporting at scheduled study visits) to identify potential cases of intussusception within 31 days after vaccination and, in a subset of 20,169 infants (10,159 received ROTARIX and 10,010 received placebo), up to one year after the first dose.
No increased risk of intussusception following administration of ROTARIX was observed within a 31-day period following any dose, and rates were comparable to the placebo group after a median of 100 days (Table 2). In a subset of 20,169 infants (10,159 received ROTARIX and 10,010 received placebo) followed up to one year after Dose 1, there were 4 cases of intussusception with ROTARIX compared with 14 cases of intussusception with placebo (RR: 0.28 [95% CI: 0.10, 0.81]). All of the infants who developed intussusception recovered without sequelae.
| CI = Confidence Interval. a Median duration after Dose 1 (follow-up visit at 30 to 90 days after Dose 2). |
|||||||
|
Confirmed Cases of Intussusception |
ROTARIX |
Placebo |
|||||
|
n = 31,673 |
n = 31,552 |
||||||
|
Within 31 days following diagnosis after any dose |
6 |
7 |
|||||
|
Relative Risk (95% CI) |
0.85 (0.30, 2.42) |
||||||
|
Within 100 days following Dose 1a |
9 |
16 |
|||||
|
Relative Risk (95% CI) |
0.56 (0.25, 1.24) |
||||||
Among vaccine recipients, there were no confirmed cases of intussusception within the 0- to 14-day period after the first dose (Table 3), which was the period of highest risk for the previously licensed oral live rhesus rotavirus-based vaccine.1
|
Day Range |
Dose 1 |
Dose 2 |
Any Dose |
|||
|
ROTARIX |
Placebo |
ROTARIX |
Placebo |
ROTARIX |
Placebo |
|
|
n = 31,673 |
n = 31,552 |
n = 29,616 |
n = 29,465 |
n = 31,673 |
n = 31,552 |
|
|
0-7 |
0 |
0 |
2 |
0 |
2 |
0 |
|
8-14 |
0 |
0 |
0 |
2 |
0 |
2 |
|
15-21 |
1 |
1 |
2 |
1 |
3 |
2 |
|
22-30 |
0 |
1 |
1 |
2 |
1 |
3 |
|
Total (0-30) |
1 |
2 |
5 |
5 |
6 |
7 |
Kawasaki Disease − Results from Controlled and Uncontrolled Clinical Studies: Kawasaki disease has been reported in 18 (0.035%) recipients of ROTARIX and 9 (0.021%) placebo recipients from 16 completed or ongoing clinical trials (Studies 1 to 8; Studies 12 to 14, NCT00425737, NCT00346892, NCT00139347; Studies 15 to 17, NCT00197210 for the 3 studies; Studies 18 and 19, NCT00334607, NCT00382772). Of the 27 cases, 5 occurred following ROTARIX in clinical trials that were either not placebo-controlled or 1:1 randomized. In placebo-controlled trials, Kawasaki disease was reported in 17 recipients of ROTARIX and 9 placebo recipients (RR: 1.71 [95% CI: 0.71, 4.38]). Three of the 27 cases were reported within 30 days post-vaccination: 2 cases (ROTARIX = 1, placebo = 1) were from placebo-controlled trials (RR: 1.00 [95% CI: 0.01, 78.35]) and one case following ROTARIX was from a non–placebo-controlled trial. Among recipients of ROTARIX, the time of onset after study dose ranged 3 days to 19 months.
Clinical Trials Comparing the Two ROTARIX Formulations
The safety of the ROTARIX liquid formulation was evaluated in 3 randomized clinical studies (Studies 9 to 11). A total of 4,223 infants received ROTARIX (liquid formulation, n = 2,507; reconstituted lyophilized formulation, n = 1,716). The racial distribution for these 3 studies was as follows: Asian 24.4%, White 63.2%, Black or African American 5.1%, and other 7.3%; 49.7% were male.
In Study 10, a concomitant vaccine administration study conducted in United States, 1,272 infants received ROTARIX (liquid formulation, n = 632; reconstituted lyophilized formulation, n = 640). The racial distribution for this study was as follows: Asian 3.3%, White 73.8%, Black or African American 11.9%, and other 10.9%; 51.5% were male.
In Studies 9 to 11, solicited general adverse reactions (cough/runny nose, diarrhea, fever, irritability/fussiness, loss of appetite and vomiting) were recorded by the parent on diary cards during the 8 days after each vaccination (day of vaccination and 7 following days). Unsolicited adverse events were assessed within 31 days following each vaccination (day of vaccination and 30 following days). SAEs were assessed through 6 months after the last dose.
Solicited Adverse Reactions: In Study 10, solicited adverse reactions among recipients of the two ROTARIX formulations are presented in the Table 4:
| G3 = Grade 3. Total vaccinated cohort = All vaccinated infants for whom safety data were available. n = Number of infants who received the specified dose. a Overall: Defined as crying more than usual. Grade 3: Crying that could not be comforted/prevented normal activity. b Grade 3 cough/runny nose: cough/runny nose that prevented daily activity. c Overall: Defined as temperature ≥100.4°F (≥38.0°C). Grade 3: Defined as temperature >103.1 F (>39.5°C). d Overall: Defined as eating less than usual. Grade 3: Defined as not eating at all. e Overall: Defined as 1 or more episodes of forceful emptying of partially digested stomach contents ≥1 hour after feeding within a day. Grade 3: ≥3 episodes of vomiting/day. f Overall: Defined as passage of 3 or more looser than normal stools within a day. Grade 3: ≥6 looser than normal stools/day. |
|||||||||||||||||||||||
|
Adverse Reactions |
Dose 1 |
Dose 2 |
|||||||||||||||||||||
|
ROTARIX (Liquid Formulation) n = 632 |
ROTARIX (Reconstituted Lyophilized Formulation) n = 640 |
ROTARIX (Liquid Formulation) n = 607 |
ROTARIX (Reconstituted Lyophilized Formulation) n = 609 |
||||||||||||||||||||
|
Overall% |
G3% |
Overall% |
G3% |
Overall% |
G3% |
Overall% |
G3% |
||||||||||||||||
|
Fussiness/irritabilitya |
70.9 |
9.2 |
71.6 |
8.8 |
72.5 |
13.7 |
70.1 |
11.8 |
|||||||||||||||
|
Cough/runny noseb |
27.2 |
0.5 |
28.1 |
1.3 |
36.9 |
3.8 |
36.5 |
3.6 |
|||||||||||||||
|
Feverc |
5.7 |
0.3 |
5.0 |
0.3 |
10.5 |
0.3 |
12.3 |
0.7 |
|||||||||||||||
|
Loss of appetited |
32.3 |
0.2 |
33.4 |
0.9 |
29.5 |
1.2 |
29.2 |
1.8 |
|||||||||||||||
|
Vomitinge |
17.4 |
2.8 |
16.4 |
3.4 |
13.7 |
4.0 |
12.8 |
3.3 |
|||||||||||||||
|
Diarrheaf |
6.2 |
0.2 |
5.6 |
0.8 |
5.6 |
0.5 |
4.3 |
0.3 |
|||||||||||||||
Unsolicited Adverse Events: Infants were monitored for unsolicited serious and non-SAEs that occurred in the 31-day period following vaccination in Studies 9 to 11. There were no notable differences in the occurrence and frequency of unsolicited adverse events between the groups.
Serious Adverse Events: During the entire course of Studies 9 to 11, SAEs occurred in 4.7% of recipients of ROTARIX liquid formulation (n = 2,507) as compared with 4.4% of ROTARIX reconstituted lyophilized formulation recipients (n = 1,716).
During the entire course of Studies 9 to 11, there was 1 fatal SAE (with diagnosis of sudden infant death syndrome) following administration of ROTARIX liquid formulation (Study 10). The SAE was assessed as not causally related to the vaccination.
Among participants in Studies 9 to 11, 2 intussusception cases were reported. One subject from Study 10 experienced intussusception 8 days after receiving the second dose of ROTARIX (reconstituted lyophilized formulation). The event was considered possibly related to ROTARIX. One subject from Study 9 experienced intussusception 133 days after receiving the second dose of ROTARIX (liquid formulation); the event was not considered related to ROTARIX. Both subjects were hospitalized, and the outcome of intussusception was reported as resolved.
6.2 Postmarketing Experience
The temporal association between vaccination with ROTARIX and intussusception was evaluated in a hospital-based active surveillance study that identified infants with intussusception at participating hospitals in Mexico. Using a self-controlled case series method,4 the incidence of intussusception during the first 7 days after receipt of ROTARIX and during the 31-day period after receipt of ROTARIX was compared with a control period. The control period was from birth to one year, excluding the pre-defined risk period (first 7 days or first 31 days post-vaccination, respectively).
Over a 2-year period, the participating hospitals provided health services to approximately 1 million infants under 1 year of age. Among 750 infants with intussusception, the relative incidence of intussusception in the 31‑day period after the first dose of ROTARIX compared with the control period was 1.96 (95.5% CI: 1.46, 2.63)]; the relative incidence of intussusception in the first 7 days after the first dose of ROTARIX compared with the control period was 6.07 (95.5% CI: 4.20, 8.63).
The Mexico study did not take into account all medical conditions that may predispose infants to intussusception. The results may not be generalizable to U.S. infants who have a lower background rate of intussusception than Mexican infants. However, if a temporal increase in the risk for intussusception following ROTARIX similar in magnitude to that observed in the Mexico study does exist in U.S. infants, it is estimated that approximately 1 to 3 additional cases of intussusception hospitalizations would occur per 100,000 vaccinated infants in the U.S. within 7 days following the first dose of ROTARIX. In the first year of life, the background rate of intussusception hospitalizations in the U.S. has been estimated to be approximately 34 per 100,000 infants.5
Other postmarketing observational studies conducted in Brazil and Australia also suggest an increased risk of intussusception within the first 7 days following the second dose of ROTARIX.2,3
Worldwide passive postmarketing surveillance data suggest that most cases of intussusception reported following ROTARIX occur in the 7-day period after the first dose.
The following adverse reactions have been identified during postapproval use of ROTARIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccination.
Gastrointestinal Disorders
Intussusception (including death), recurrent intussusception (including death), hematochezia, gastroenteritis with vaccine viral shedding in infants with SCID.
Blood and Lymphatic System Disorders
Idiopathic thrombocytopenic purpura.
Vascular Disorders
Kawasaki disease.
General Disorders and Administration Site Conditions
Maladministration.
7. Drug Interactions
7.1 Immunosuppressive Therapies
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune response to ROTARIX. [See Warnings and Precautions (5.3).]
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of ROTARIX in infants younger than 6 weeks or older than 24 weeks of age have not been evaluated.
The effectiveness of ROTARIX in pre-term infants has not been established. Safety data are available in pre-term infants (ROTARIX = 134, placebo = 120) with a reported gestational age ≤36 weeks. These pre-term infants were followed for SAEs up to 30 to 90 days after Dose 2. SAEs were observed in 5.2% of recipients of ROTARIX as compared with 5.0% of placebo recipients. No deaths or cases of intussusception were reported in this population.
11. Rotarix Description
ROTARIX (Rotavirus Vaccine, Live, Oral), for oral administration, is a live, attenuated rotavirus vaccine derived from the human 89-12 strain which belongs to G1P[8] type. The rotavirus vaccine strain is propagated on Vero cells.
There are two formulations of ROTARIX: a reconstituted lyophilized formulation (supplied in a vial and oral dosing applicator presentation) and a liquid formulation (supplied in an oral dosing applicator only presentation).
Formulation for the Vial and Oral Dosing Applicator Presentation
After reconstitution of the lyophilized vaccine component, each 1 mL dose of the ROTARIX formulation for the vial and oral dosing applicator presentation contains at least 106.0 median Cell Culture Infective Dose (CCID50) of live, attenuated rotavirus.
The lyophilized vaccine component of this ROTARIX formulation contains amino acids, dextran, Dulbecco’s Modified Eagle Medium (DMEM), sorbitol, and sucrose. DMEM contains the following ingredients: sodium chloride, potassium chloride, magnesium sulfate, ferric (III) nitrate, sodium dihydrogen phosphate, sodium pyruvate, D-glucose, concentrated vitamin solution, L-cystine, L-tyrosine, amino acids solution, L-glutamine, calcium chloride, and sodium hydrogenocarbonate.
In the manufacturing process, porcine-derived materials are used. Porcine circovirus type 1 (PCV-1) is present in this ROTARIX formulation. PCV-1 is not known to cause disease in humans.
The liquid diluent contains calcium carbonate, sterile water, and xanthan. The diluent includes an antacid component (calcium carbonate) to protect the vaccine during passage through the stomach and prevent its inactivation due to the acidic environment of the stomach.
This ROTARIX formulation is available in single-dose vials of lyophilized vaccine, accompanied by a prefilled oral dosing applicator of liquid diluent [see How Supplied/Storage and Handling (16)]. The tip caps of the prefilled oral dosing applicators contain natural rubber latex; the vial stoppers are not made with natural rubber latex.
This ROTARIX formulation contains no preservatives.
Formulation for the Oral Dosing Applicator Only Presentation
Each 1.5 mL dose of the ROTARIX formulation for the oral dosing applicator only presentation contains at least 106.0 CCID50 of live, attenuated rotavirus.
This ROTARIX formulation contains disodium adipate, Dulbecco’s Modified Eagle Medium (DMEM), sucrose, and sterile water. DMEM contains the following ingredients: sodium chloride, potassium chloride, magnesium sulfate, ferric (III) nitrate, sodium dihydrogen phosphate, sodium pyruvate, D-glucose, concentrated vitamin solution, L-cystine, L-tyrosine, amino acids solution, L-glutamine, calcium chloride, and sodium hydrogenocarbonate.
This ROTARIX formulation contains an antacid component (disodium adipate) to protect the vaccine during passage through the stomach and prevent its inactivation due to the acidic environment of the stomach.
This ROTARIX formulation is available in single-dose prefilled oral dosing applicator [see How Supplied/Storage and Handling (16)]. The tip caps of the prefilled oral dosing applicators contain natural rubber latex.
This ROTARIX formulation contains no preservatives.
12. Rotarix - Clinical Pharmacology
12.1 Mechanism of Action
The exact immunologic mechanism by which ROTARIX protects against rotavirus gastroenteritis is unknown [see Clinical Studies (14.4)]. ROTARIX contains a live, attenuated human rotavirus that replicates in the small intestine and induces immunity.
12.2 Pharmacodynamics
Shedding and Transmission
A prospective, randomized, double-blind, placebo-controlled study was performed in the Dominican Republic in twins within the same household to assess whether transmission of vaccine virus occurs from a vaccinated infant to a non-vaccinated infant (Study 20; NCT00396630). One hundred pairs of healthy twins 6 to 14 weeks of age (gestational age ≥32 weeks) were randomized with one twin to receive ROTARIX (n = 100) and the other twin to receive placebo (n = 100). Twenty subjects in each arm were excluded for reasons such as having rotavirus antibody at baseline. Stool samples were collected on the day of or 1 day prior to each dose, as well as 3 times weekly for 6 consecutive weeks after each dose of ROTARIX or placebo. Transmission was defined as presence of the vaccine virus strain in any stool sample from a twin receiving placebo.
Transmitted vaccine virus was identified in 15 of 80 twins receiving placebo (18.8% [95% CI: 10.9, 29.0]). Median duration of the rotavirus shedding was 10 days in twins who received ROTARIX as compared with 4 days in twins who received placebo in whom the vaccine virus was transmitted. In the 15 twins who received placebo, no gastrointestinal symptoms related to transmitted vaccine virus were observed.
14. Clinical Studies
There are two formulations of ROTARIX: a reconstituted lyophilized formulation (supplied in a vial and oral dosing applicator presentation) and a liquid formulation (supplied in an oral dosing applicator only presentation) [see Description (11)]. Efficacy was evaluated using the reconstituted lyophilized formulation [see Clinical Studies (14.1, 14.2, 14.3)]. These data are relevant to the liquid formulation because both formulations contain the same live, attenuated rotavirus strain and are manufactured using a similar process.
14.1 Efficacy Studies
The data demonstrating the efficacy of ROTARIX in preventing rotavirus gastroenteritis come from 24,163 infants randomized in two placebo-controlled studies conducted in 17 countries in Europe and Latin America (Studies 5 and 8). In these studies, oral polio vaccine (OPV) was not coadministered; however, other routine childhood vaccines could be concomitantly administered. Breastfeeding was permitted in both studies.
A randomized, double-blind, placebo-controlled study was conducted in 6 European countries (Study 5). A total of 3,994 infants were enrolled to receive ROTARIX (n = 2,646) or placebo (n = 1,348). Vaccine or placebo was given to healthy infants as a 2-dose series with the first dose administered orally from 6 through 14 weeks of age followed by one additional dose administered at least 4 weeks after the first dose. The 2-dose series was completed by 24 weeks of age. For both vaccination groups, 98.3% of infants were White and 53% were male.
The clinical case definition of rotavirus gastroenteritis was an episode of diarrhea (passage of 3 or more loose or watery stools within a day), with or without vomiting, where rotavirus was identified in a stool sample. Severity of gastroenteritis was determined by a clinical scoring system, the Vesikari scale, assessing the duration and intensity of diarrhea and vomiting, the intensity of fever, use of rehydration therapy, or hospitalization for each episode. Scores range from 0 to 20, where higher scores indicate greater severity. An episode of gastroenteritis with a score of 11 or greater was considered severe.6
The primary efficacy endpoint was prevention of any grade of severity of rotavirus gastroenteritis caused by naturally occurring rotavirus from 2 weeks after the second dose through one rotavirus season (according to protocol, ATP). Other efficacy evaluations included prevention of severe rotavirus gastroenteritis, as defined by the Vesikari scale, and reductions in hospitalizations due to rotavirus gastroenteritis and all-cause gastroenteritis regardless of presumed etiology. Analyses were also done to evaluate the efficacy of ROTARIX against rotavirus gastroenteritis among infants who received at least one vaccination (total vaccinated cohort, TVC).
Efficacy of ROTARIX against any grade of severity of rotavirus gastroenteritis through one rotavirus season was 87.1% (95% CI: 79.6, 92.1); TVC efficacy was 87.3% (95% CI: 80.3, 92.0). Efficacy against severe rotavirus gastroenteritis through one rotavirus season was 95.8% (95% CI: 89.6, 98.7); TVC efficacy was 96.0% (95% CI: 90.2, 98.8) (Table 5). The protective effect of ROTARIX against any grade of severity of rotavirus gastroenteritis observed immediately following Dose 1 administration and prior to Dose 2 was 89.8% (95% CI: 8.9, 99.8).
Efficacy of ROTARIX in reducing hospitalizations for rotavirus gastroenteritis through one rotavirus season was 100% (95% CI: 81.8, 100); TVC efficacy was 100% (95% CI: 81.7, 100) (Table 5). ROTARIX reduced hospitalizations for all-cause gastroenteritis regardless of presumed etiology by 74.7% (95% CI: 45.5, 88.9).
| RV GE = Rotavirus gastroenteritis; CI = Confidence Interval. a ATP analysis includes all infants in the efficacy cohort who received two doses of vaccine according to randomization. b TVC analysis includes all infants in the efficacy cohort who received at least one dose of vaccine or placebo. c Severe gastroenteritis defined as ≥11 on the Vesikari scale. d Statistically significant versus placebo (P <0.001). |
||||
|
Infants in Cohort |
According to Protocola |
Total Vaccinated Cohortb |
||
|
ROTARIX |
Placebo |
ROTARIX |
Placebo |
|
|
n = 2,572 |
n = 1,302 |
n = 2,646 |
n = 1,348 |
|
|
Gastroenteritis cases | ||||
|
Any severity |
24 |
94 |
26 |
104 |
|
Severec |
5 |
60 |
5 |
64 |
|
Efficacy estimate against RV GE | ||||
|
Any severity |
87.1%d |
87.3%d |
||
|
(95% CI) |
(79.6, 92.1) |
(80.3, 92.0) |
||
|
Severec |
95.8%d |
96.0%d |
||
|
(95% CI) |
(89.6, 98.7) |
(90.2, 98.8) |
||
|
Cases of hospitalization due to RV GE |
0 |
12 |
0 |
12 |
|
Efficacy in reducing hospitalizations due to RV GE |
100%d |
100%d |
||
|
(95% CI) |
(81.8, 100) |
(81.7, 100) |
||
A randomized, double-blind, placebo-controlled study was conducted in 11 countries in Latin America and Finland (Study 8). A total of 63,225 infants received ROTARIX (n = 31,673) or placebo (n = 31,552). An efficacy subset of these infants consisting of 20,169 infants from Latin America received ROTARIX (n = 10,159) or placebo (n = 10,010). Vaccine or placebo was given to healthy infants as a 2-dose series with the first dose administered orally from 6 through 13 weeks of age followed by one additional dose administered at least 4 weeks after the first dose. The 2−dose series was completed by 24 weeks of age. For both vaccination groups, the racial distribution of the efficacy subset was as follows: Hispanic 85.8%, White 7.9%, Black 1.1%, and other 5.2%; 51% were male.
The clinical case definition of severe rotavirus gastroenteritis was an episode of diarrhea (passage of 3 or more loose or watery stools within a day), with or without vomiting, where rotavirus was identified in a stool sample, requiring hospitalization and/or rehydration therapy equivalent to World Health Organization (WHO) plan B (oral rehydration therapy) or plan C (intravenous rehydration therapy) in a medical facility.
The primary efficacy endpoint was prevention of severe rotavirus gastroenteritis caused by naturally occurring rotavirus from 2 weeks after the second dose through one year (ATP). Analyses were done to evaluate the efficacy of ROTARIX against severe rotavirus gastroenteritis among infants who received at least one vaccination (TVC). Reduction in hospitalizations due to rotavirus gastroenteritis was also evaluated (ATP).
Efficacy of ROTARIX against severe rotavirus gastroenteritis through one year was 84.7% (95% CI: 71.7, 92.4); TVC efficacy was 81.1% (95% CI: 68.5, 89.3) (Table 6).
Efficacy of ROTARIX in reducing hospitalizations for rotavirus gastroenteritis through one year was 85.0% (95% CI: 69.6, 93.5); TVC efficacy was 80.8% (95% CI: 65.7, 90.0) (Table 6).
| RV GE = Rotavirus gastroenteritis; CI = Confidence Interval. a ATP analysis includes all infants in the efficacy cohort who received two doses of vaccine according to randomization. b TVC analysis includes all infants in the efficacy cohort who received at least one dose of vaccine or placebo. c Statistically significant versus placebo (P <0.001). |
|||||||||
|
Infants in Cohort |
According to Protocola |
Total Vaccinated Cohortb |
|||||||
|
ROTARIX |
Placebo |
ROTARIX |
Placebo |
||||||
|
n = 9,009 |
n = 8,858 |
n = 10,159 |
n = 10,010 |
||||||
|
Gastroenteritis cases | |||||||||
|
Severe |
12 |
77 |
18 |
94 |
|||||
|
Efficacy estimate against RV GE | |||||||||
|
Severe |
84.7%c |
81.1%c |
|||||||
|
(95% CI) |
(71.7, 92.4) |
(68.5, 89.3) |
|||||||
|
Cases of hospitalization due to RV GE |
9 |
59 |
14 |
72 |
|||||
|
Efficacy in reducing hospitalizations due to RV GE |
85.0%c |
80.8%c |
|||||||
|
(95% CI) |
(69.6, 93.5) |
(65.7, 90.0) |
|||||||
14.2 Efficacy through Two Rotavirus Seasons
The efficacy of ROTARIX persisting through two rotavirus seasons was evaluated in two studies.
In the European study (Study 5), the efficacy of ROTARIX against any grade of severity of rotavirus gastroenteritis through two rotavirus seasons was 78.9% (95% CI: 72.7, 83.8). Efficacy in preventing any grade of severity of rotavirus gastroenteritis cases occurring only during the second season post-vaccination was 71.9% (95% CI: 61.2, 79.8). The efficacy of ROTARIX against severe rotavirus gastroenteritis through two rotavirus seasons was 90.4% (95% CI: 85.1, 94.1). Efficacy in preventing severe rotavirus gastroenteritis cases occurring only during the second season post-vaccination was 85.6% (95% CI: 75.8, 91.9).
The efficacy of ROTARIX in reducing hospitalizations for rotavirus gastroenteritis through two rotavirus seasons was 96.0% (95% CI: 83.8, 99.5).
In the Latin American study (Study 8), the efficacy of ROTARIX against severe rotavirus gastroenteritis through two years was 80.5% (95% CI: 71.3, 87.1). Efficacy in preventing severe rotavirus gastroenteritis cases occurring only during the second year post-vaccination was 79.0% (95% CI: 66.4, 87.4). The efficacy of ROTARIX in reducing hospitalizations for rotavirus gastroenteritis through two years was 83.0% (95% CI: 73.1, 89.7).
The efficacy of ROTARIX beyond the second season post-vaccination was not evaluated.
14.3 Efficacy against Specific Rotavirus Types
The type-specific efficacy against any grade of severity and severe rotavirus gastroenteritis caused by G1P[8], G3P[8], G4P[8], G9P[8], and combined non-G1 (G2, G3, G4, G9) types was statistically significant through one year. Additionally, type-specific efficacy against any grade of severity and severe rotavirus gastroenteritis caused by G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], and combined non-G1 (G2, G3, G4, G9) types was statistically significant through two years (Table 7).
| CI = Confidence Interval; NS = Not significant. a Statistical analyses done by G type; if more than one rotavirus type was detected from a rotavirus gastroenteritis episode, the episode was counted in each of the detected rotavirus type categories. b Statistically significant versus placebo (P <0.05). c The P genotype was not typeable for one episode. d P[8] genotype was not detected in one episode. e Two cases of G12P[8] were isolated in the second season (one in each group). |
||||||
|
Type Identifieda |
Through One Rotavirus Season |
Through Two Rotavirus Seasons |
||||
|
Number of Cases |
Number of Cases | |||||
|
ROTARIX |
Placebo |
% Efficacy |
ROTARIX |
Placebo |
% Efficacy |
|
|
n = 2,572 |
n = 1,302 |
(95% CI) |
n = 2,572 |
n = 1,302 |
(95% CI) |
|
|
Any Grade of Severity |
||||||
|
G1P[8] |
4 |
46 |
95.6%b (87.9, 98.8) |
18 |
89c,d |
89.8%b (82.9, 94.2) |
|
G2P[4] |
3 |
4c |
NS |
14 |
17c |
58.3%b (10.1, 81.0) |
|
G3P[8] |
1 |
5 |
89.9%b (9.5, 99.8) |
3 |
10 |
84.8%b (41.0, 97.3) |
|
G4P[8] |
3 |
13 |
88.3%b (57.5, 97.9) |
6 |
18 |
83.1%b (55.6, 94.5) |
|
G9P[8] |
13 |
27 |
75.6%b (51.1, 88.5) |
38 |
71d |
72.9%b (59.3, 82.2) |
|
Combined non-G1 (G2, G3, G4, G9, G12) typese |
20 |
49 |
79.3%b (64.6, 88.4) |
62 |
116 |
72.9%b (62.9, 80.5) |
|
Severe |
||||||
|
G1P[8] |
2 |
28 |
96.4%b (85.7, 99.6) |
4 |
57 |
96.4%b (90.4, 99.1) |
|
G2P[4] |
1 |
2c |
NS |
2 |
7c |
85.5%b (24.0, 98.5) |
|
G3P[8] |
0 |
5 |
100%b (44.8, 100) |
1 |
8 |
93.7%b (52.8, 99.9) |
|
G4P[8] |
0 |
7 |
100%b (64.9, 100) |
1 |
11 |
95.4%b (68.3, 99.9) |
|
G9P[8] |
2 |
19 |
94.7%b (77.9, 99.4) |
13 |
44d |
85.0%b (71.7, 92.6) |
|
Combined non-G1 (G2, G3, G4, G9, G12) typese |
3 |
33 |
95.4%b (85.3, 99.1) |
17 |
70 |
87.7%b (78.9, 93.2) |
14.4 Immunogenicity
A relationship between antibody responses to rotavirus vaccination and protection against rotavirus gastroenteritis has not been established. Seroconversion was defined as the appearance of anti-rotavirus IgA antibodies (concentration ≥20 U/mL) post-vaccination in the serum of infants previously negative for rotavirus.
In 2 safety and efficacy studies (Studies 5 and 8), one to two months after a 2-dose series, 86.5% of 787 recipients of ROTARIX reconstituted lyophilized formulation seroconverted compared with 6.7% of 420 placebo recipients, and 76.8% of 393 recipients of ROTARIX reconstituted lyophilized formulation seroconverted compared with 9.7% of 341 placebo recipients, respectively.
Immunogenicity of a 2-dose series of ROTARIX liquid formulation was evaluated in Study 9. This study compared seroconversion rates and Geometric Mean Concentrations (GMCs) following administration of the ROTARIX liquid formulation (n = 984) or the ROTARIX reconstituted lyophilized formulation (n = 329). The primary analyses demonstrated non-inferiority of IgA seroconversion rates and GMCs at 1 to 2 months post-vaccination for the ROTARIX liquid formulation group compared to the ROTARIX reconstituted lyophilized formulation group (Lower Limit of the 95% CI for the difference in seroconversion rates ≥-10%; Lower Limit of the 95% CI for the GMC ratio ≥0.67).
In Study 10, 3 months after a 2-dose series, the percentage of subjects with anti-rotavirus IgA antibodies (concentration ≥20 U/mL) was comparable after administration of the ROTARIX liquid formulation (76.3% of 417) and the ROTARIX reconstituted lyophilized formulation (78.9% of 426).
14.5 Concomitant Vaccine Administration
In clinical trials, ROTARIX was administered concomitantly with U.S.-licensed and non–U.S.-licensed vaccines. In a U.S. concomitant vaccine administration study (Study 18) using ROTARIX (reconstituted lyophilized formulation) in 484 infants, there was no evidence of interference in the immune responses to any of the antigens when PEDIARIX [Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus Vaccine], a U.S.−licensed 7−valent pneumococcal conjugate vaccine (Wyeth Pharmaceuticals Inc.), and a U.S.−licensed Haemophilus b conjugate vaccine (Sanofi Pasteur SA) were concomitantly administered with ROTARIX as compared with separate administration of ROTARIX.
In a concomitant vaccine administration study (Study 10) in 1,272 infants, non-inferiority was demonstrated regarding the immune response to each of the antigens in PEDIARIX, HIBERIX [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)], and a U.S.‑licensed, 13‑valent pneumococcal conjugate vaccine (Pfizer Inc.) when concomitantly administered with the ROTARIX liquid formulation as compared to when concomitantly administered with the ROTARIX reconstituted lyophilized formulation.
15. References
- 1.
- Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572.
- 2.
- Carlin JB, Macartney KK, Lee KJ, et al. Intussusception Risk and Disease Prevention Associated with Rotavirus Vaccines in Australia’s National Immunization Program. CID. 2013;57(10):1427-1434.
- 3.
- Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception Risk and Health Benefits of Rotavirus Vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283-2292.
- 4.
- Farrington CP, Whitaker HJ, Hocine MN, et al. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10(1):3–16.
- 5.
- Tate JE, Simonsen L, Viboud C, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121:e1125-e1132.
- 6.
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for severity of diarrheal episodes. Scand J Infect Dis. 1990;22:259-267.
16. How is Rotarix supplied
16.1 ROTARIX Vial and Oral Dosing Applicator Presentation
The ROTARIX vial and oral dosing applicator presentation is supplied as single-dose vials of lyophilized vaccine component, accompanied by a prefilled oral dosing applicator of liquid diluent (1 mL) with a plunger stopper, and a transfer adapter for reconstitution.
Supplied as an outer package of 10 doses (NDC 58160-854-52) containing:
- •
- Inner package of 10 vials of lyophilized vaccine component (NDC 58160-851-10)
- o
- Single vial of lyophilized vaccine component (NDC 58160-851-01)
- •
- Oral dosing applicator of diluent (NDC 58160-853-02) (10 applicators)
Storage before Reconstitution
- •
- Lyophilized vaccine component in vials: Store refrigerated at 2° to 8°C (36° to 46°F). Protect vials from light.
- •
- Diluent in oral dosing applicators: Store refrigerated at 2° to 8°C (36° to 46°F) or at a controlled room temperature up to 25°C (77°F). Do not freeze. Discard if the diluent has been frozen.
Storage after Reconstitution
ROTARIX (vial and oral dosing applicator presentation) should be administered within 24 hours of reconstitution. After reconstitution, store refrigerated at 2° to 8°C (36° to 46°F) or at a controlled room temperature up to 25°C (77°F). Discard the reconstituted vaccine in biological waste container if not used within 24 hours. Do not freeze. Discard if the reconstituted vaccine has been frozen.
16.2 ROTARIX Oral Dosing Applicator Only Presentation
ROTARIX oral dosing applicator only presentation is supplied as a single, 1.5-mL dose in a prefilled oral dosing applicator with a plunger stopper (NDC 58160-740-02) in a carton of 10 (NDC 58160-740-21).
Storage
Store refrigerated at 2° to 8℃ (36° to 46°F). Do not freeze. Discard if the vaccine has been frozen. Keep in original package to protect from light.
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Information). Patient labeling is provided as a tear-off leaflet at the end of this full prescribing information.
Provide the following information to the parent or guardian:
- •
- Inform of the potential benefits and risks of immunization with ROTARIX, and of the importance of completing the immunization series.
- •
- Inform about the potential for adverse reactions that have been temporally associated with administration of ROTARIX or other vaccines containing similar components.
- •
- Instruct to immediately report any signs and/or symptoms of intussusception to their healthcare provider.
- •
- Give the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
ROTARIX, PEDIARIX, and HIBERIX are owned by or licensed to the GSK group of companies. The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to the GSK group of companies. The makers of these brands are not affiliated with and do not endorse the GSK group of companies or its products.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License 1617
Distributed by GlaxoSmithKline
Durham, NC 27701
©2024 GSK group of companies or its licensor.
RTX:20PI
PRINCIPAL DISPLAY PANEL
NDC 58160-854-52
ROTARIX
Rotavirus Vaccine, Live, Oral
Rx only
For ORAL USE ONLY. Do not inject.
For Use from 6 to 24 Weeks of Age
Contents (see bottom panel for storage instructions):
10 Vials of Lyophilized Vaccine
10 Prefilled Oral Dosing Applicators of Diluent
10 Transfer Adapters for Reconstitution
FOR ORAL USE
Lyophilized Vaccine and Diluent are Made in Belgium
©2019 GSK group of companies or its licensor.
- Rev. 3/19
- 497103
PRINCIPAL DISPLAY PANEL
NDC 58160-740-21
ROTARIX
Rotavirus Vaccine, Live, Oral
FOR ORAL USE ONLY.
Rx only
For ORAL USE ONLY.
Do not inject.
Do not reconstitute.
For Use from 6 to 24 Weeks of Age
Contents (10 doeses of ROTARIX:
10 Single-Dose Prefilled Oral Dosing Applicators each containing one dose of 1.5 mL
Made in Belgium
©2022 GSK group of companies or its licensor.
- Rev. 9/22
509532
| ROTARIX
rotavirus vaccine, live, oral kit |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| ROTARIX
rotavirus vaccine, live, oral solution |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Biologicals SA (372748392) |
More about Rotarix (rotavirus vaccine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: viral vaccines