Paragard: Package Insert / Prescribing Info
Package insert / product label
Generic name: copper
Dosage form: intrauterine device
Drug class: Miscellaneous vaginal agents
J Code (medical billing code): J7300 (each)
Medically reviewed by Drugs.com. Last updated on Aug 19, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
PARAGARD (intrauterine copper contraceptive)
Initial U.S. Approval: 1984
Recent Major Changes
Dosing and Administration (2)……………………………………06/2024
Indications and Usage for Paragard
Paragard is a copper-containing intrauterine system (IUS) indicated for prevention of pregnancy in females of reproductive potential for up to 10 years. (1)
Paragard Dosage and Administration
- Insert a single Paragard at the fundus of the uterine cavity. Remove Paragard no later than 10 years from the date of insertion. (2.1)
- Insert and remove Paragard only if you are a healthcare provider trained on these procedures. (2.1)
- See the Full Prescribing Information for recommended timing of insertion preparation instructions, insertion procedures, postplacement management, and instructions on removing Paragard. (2.2, 2.3, 2.4, 2.5, 2.6)
- Following the insertion, examine the patient after her first menses to confirm Paragard is still in place. (2.5)
Dosage Forms and Strengths
One sterile, T-frame IUS containing copper consisting of a T-shaped polyethylene frame with a total exposed copper surface area of 380 ± 23 mm², (approximately 176 mg of wire wrapped around the vertical stem and an approximately 68.7 mg collar placed on each side of the horizontal arm) packaged with a sterile pre-assembled inserter as a single-use, disposable device in a tray. (3, 16)
Contraindications
- Pregnancy or suspicion of pregnancy (4)
- Abnormalities of the uterus resulting in distortion of the uterine cavity (4)
- Acute pelvic inflammatory disease (PID) (4)
- Postpartum endometritis or postabortal endometritis in past 3 months (4)
- Known or suspected uterine or cervical malignancy (4)
- Uterine bleeding of unknown etiology (4)
- Untreated acute cervicitis or vaginitis or other lower genital tract infection (4)
- Conditions associated with increased susceptibility to pelvic infections (4)
- Wilson's disease (4)
- A previously placed IUD or IUS that has not been removed (4)
- Hypersensitivity to any component of Paragard including copper or any trace elements present in the copper components of Paragard (4)
Warnings and Precautions
- Ectopic Pregnancy: Promptly evaluate women who become pregnant for ectopic pregnancy while using Paragard. (5.1)
- Risks with Intrauterine Pregnancy: Increased risk of spontaneous abortion, septic abortion, premature delivery, sepsis, septic shock and death if pregnancy occurs. Remove Paragard if pregnancy occurs with Paragard in place. (5.2)
- Sepsis: Group A streptococcal infection has been reported; strict aseptic technique is essential during insertion (5.3)
- Pelvic Inflammatory Disease (PID) and Endometritis: Before using Paragard, consider the risks of PID and endometritis. Promptly assess and treat patients with signs and symptoms of PID. (5.4)
- Embedment: Surgical removal may be necessary. (5.5)
- Perforation: May reduce contraceptive effectiveness and require surgery. Risk is increased if inserted in lactating women and may be increased if inserted in women with fixed, retroverted uteri or noninvoluted uteri. (5.6)
- Expulsion: Partial or complete expulsion may occur. Remove a partially expelled Paragard. (5.7)
- Bleeding patterns: May be altered and result in heavier and longer bleeding with spotting. (5.9)
- MRI Safety Information: Patients using Paragard can be safely scanned with MRI only under certain conditions. (5.10)
Adverse Reactions/Side Effects
Adverse reactions reported in clinical trials include: anemia, backache, dysmenorrhea, dyspareunia, expulsion (complete or partial), prolonged menstrual flow, menstrual spotting, pain and cramping, and vaginitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact CooperSurgical, Inc. at 1-877-727-2427 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
Full Prescribing Information
1. Indications and Usage for Paragard
Paragard is indicated for prevention of pregnancy in females of reproductive potential for up to 10 years.
2. Paragard Dosage and Administration
2.1 Important Dosage and Administration Instructions
- Paragard should only be inserted by a healthcare provider trained in Paragard's insertion procedures, because insertion for Paragard is different from that used for other intrauterine systems. Healthcare providers should become thoroughly familiar with the product, product educational materials, product insertion instructions, and prescribing information before attempting insertion of Paragard.
- Insert one Paragard at the fundus of the uterine cavity [see Dosage and Administration (2.4)].
- Remove Paragard on or before 10 years from the date of insertion [see Dosage and Administration (2.6)].
- May replace Paragard at the time of removal with a new Paragard if continued contraceptive protection is desired.
- Before considering use of Paragard, make sure that the female is an appropriate candidate for Paragard. Exclude pregnancy (consider the possibility of ovulation and conception) prior to use [see Contraindications (4) and Warnings and Precautions (5.2)].
2.2 Timing of Insertion
Refer to Table 1 for recommended timing of Paragard insertion.
|
Note: The inserter provided with Paragard (see Figure 1) and the Insertion Procedure described in subsection 2.4 are not applicable for immediate insertion after childbirth. For immediate insertion post childbirth, remove the inserter and Paragard from the tray, move the button forward and then completely back to release the Paragard unit from the inserter and insert Paragard. |
|
| Clinical Situation | Recommended Timing of Paragard Insertion |
| 1. Start Paragard in females not currently using contraception | At any time during the menstrual cycle. |
| 2. Switch to Paragard from an oral, transdermal, or vaginal form of hormonal contraception or an injectable progestin contraceptive |
At any time during the menstrual cycle; discontinue the previous method. |
| 3. Switch to Paragard from a contraceptive implant or other intrauterine system | Same day the implant or IUS is removed (insert at any time during the menstrual cycle). |
|
4. Insert Paragard after abortion or miscarriage | Immediately after abortion, although immediate placement has a slightly higher risk of expulsion than placement at other times. Insertion after second trimester abortion is associated with a higher risk of expulsion than insertion after a first trimester abortion. |
|
5. Insert Paragard after Childbirth | May insert immediately postpartum. Insertion before uterine involution is complete, which may not occur until the second postpartum month, has been associated with increased risk of expulsion [see Warnings and Precautions (5.6,5.7)]. There appears to be an increased risk of perforation in lactating women [see Warnings and Precautions (5.6)]. |
2.3 Preparation Instructions
Read the Preparation Instructions and Insertion Procedure before initiating Paragard insertion.
Before insertion:
- Do NOT remove the inserter from the tray before the arms are loaded into the insertion tube. The tray will be used to fold the Paragard arms down and load the IUS into the inserter.
- Use strict aseptic techniques throughout preparation [see Warnings and Precautions (5.4)].
- Prepare placement tools (e.g., speculum, cotton swab, tenaculum, uterine sound, scissors, and forceps).
- Consider use of an analgesic.
- Establish the size and position of the uterus by performing a bi-manual examination.
- Insert a speculum and, using a cotton swab, cleanse the cervix and vagina with an antiseptic solution.
- Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
- Gently insert a sterile uterine sound to measure the depth of the uterine cavity. The uterus should sound to a depth of 6 to 9 cm except when inserting Paragard immediately postabortion or immediately postpartum.
- Insertion of Paragard may be associated with pain and/or bleeding, vasovagal reactions (e.g. syncope, bradycardia), or seizure, especially in patients with a predisposition to these conditions.
- Insertion into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation.
- If cervical stenosis is encountered, avoid undue force. Dilators and analgesia/local anesthesia may be helpful in this situation.
2.4 Insertion Procedure
Follow the insertion instructions exactly as described to ensure proper placement and avoid early release of Paragard from the inserter.
- Use strict aseptic techniques throughout the insertion procedure [see Warnings and Precautions (5.4)].
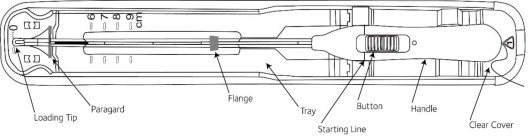
Step 1: Opening the Sterile Paragard Package
-
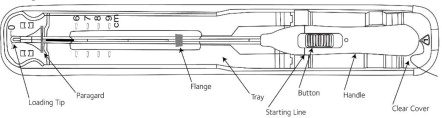
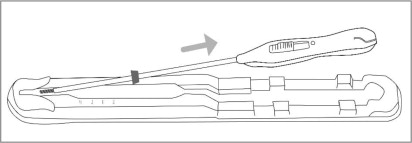
Place the package containing Paragard (face-up) on a flat sterile field (see Figure 1). Open the pouch from the handle end where the arrow on the Placement Guide says “OPEN”.
- Remove the clear cover from the tray (not shown).
- Confirm that the top of the button is located at the starting line on the handle prior to loading Paragard.
- The inserter should remain in the tray until Paragard T-Arms are loaded.
Do not slide the button on the handle before folding the arms in the tray.
Do not repeatedly slide the button forward and back as this may cause slack in the threads and may result in an unsuccessful placement.
Step 2: Loading Paragard into the Inserter
-
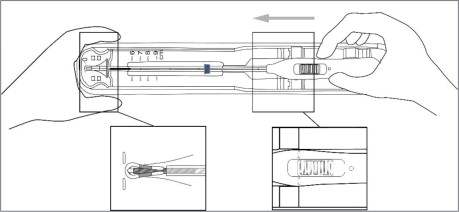
Using sterile gloves, place one hand on the distal end of the tray and the other on the inserter handle. Slide the handle completely forward so that the Paragard advances into the Loading Tip folding the T-Arms of Paragard against the stem (see Figure 3).
Figure 3: Folding Tips of T-Arms of Paragard using Loading Tip
- Once the T-Arms are folded against the stem, slide the button on the handle completely forward to advance the insertion tube over the tips of the T-Arms (see Figure 4). Only the tips of the T-Arms should be in the insertion tube. Do not advance beyond the copper collars.
IMPORTANT: Do not leave the T-Arms of Paragard bent for more than 5 minutes, as the arms may not open properly.
Figure 4: Inserting Tips of T-Arms into Insertion Tube
Step 3: Adjusting the Flange
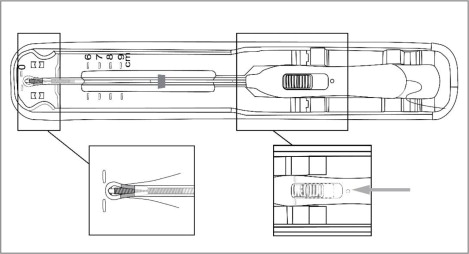
- Once the above steps are completed and Paragard is in the insertion tube, adjust the blue flange. The tray is marked with centimeters and can be used to set the flange to the correct depth (see Figure 5).
-
Adjust the flange so the distance from the top of Paragard (where it protrudes from the inserter) to the top of the flange is equal to the pre-measured uterine depth.
Step 4: Removing Inserter from Tray
- Ensure the button remains in the forward position.
- To remove the inserter from the tray, gently lift the handle out of the tray, then gently slide the inserter back and lift out of the tray. (see Figure 6).
- Upon removal from the tray, verify and rotate the blue flange as needed so that the horizontal arms of Paragard and the long axis of the blue flange and handle lie in the same horizontal plane to ensure the arms open in the proper direction.
-
Confirm that both T-Arms are captured within the insertion tube.
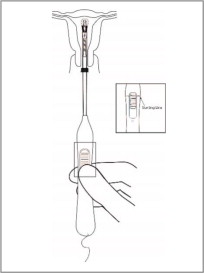
Step 5: Inserting Paragard to the Fundus
- To orient the uterus in an axial position, apply gentle traction to the tenaculum. While holding the button forward, pass the loaded inserter through the cervical canal until the Paragard reaches the fundus of the uterus. This will ensure placement of Paragard at the highest possible position within the uterus.
-
The blue flange should be at the cervix in the horizontal plane. The button should remain in the forward position (see Figure 7).
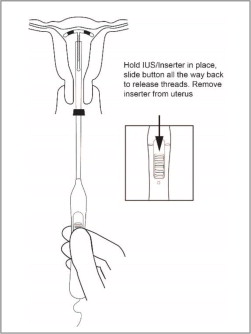
Step 6: Releasing Paragard and Withdrawing Inserter
- Release the arms of Paragard by holding the handle steady and sliding the button all the way down (and you may feel a click). Do not stop at the starting line as it is not used for deployment.
-
This releases the threads and the T-Arms of Paragard high in the uterine fundus (see Figure 8).
- Gently and slowly withdraw the inserter from the uterus and cervical canal.
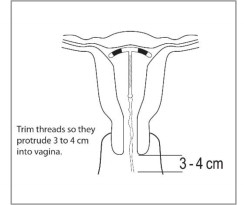
- Only the threads should be visibly protruding from the cervix (see Figure 9). Trim the threads so that 3 to 4 cm protrude into the vagina.
- Measure and note the length of threads, date of placement and Paragard lot number.
- Discard the used inserter – do not attempt to re-use the inserter because it is a single use device.
-
If you suspect that Paragard is not in the correct position, check placement (with ultrasound, if necessary). If Paragard is not positioned completely within the uterus, remove it and replace it with a new Paragard. Do not reinsert an expelled or partially expelled Paragard.
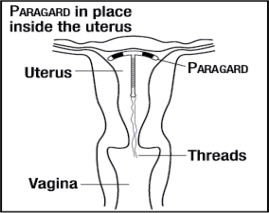
Figure 9: Appropriate Paragard Placement in Uterus
2.5 Postplacement Management of Paragard
Following placement:
- Examine the female after her first menses to confirm that Paragard is still in place. You should be able to visualize or feel only the threads. The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage.
- If you cannot find the threads in the vagina, check that Paragard is still in the uterus. The threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Paragard.
- Remove Paragard if it has been partially expelled or perforated the uterus [see Warnings and Precautions (5.6, 5.7)].
Do not reinsert a used Paragard.
2.6 Removal of Paragard
Timing of Removal
- Paragard can be removed at any time prior to 10 years after insertion.
- Remove Paragard no later than 10 years after insertion. A new Paragard can be inserted at the time of removal if continued contraceptive protection is desired.
Removal Instructions
- Use a speculum and visualize the cervix.
- Remove Paragard with forceps, pulling gently on the exposed threads. The arms of Paragard will fold upwards as it is withdrawn from the uterus.
- If removal cannot be accomplished by gentle pulling, consider checking Paragard location and assess for embedment and perforation (with imaging, if necessary).
- Breakage or embedment of Paragard in the myometrium can make removal difficult [see Warnings and Precautions, (5.5)]. IUS breakage may be associated with removal. Analgesia, paracervical anesthesia, cervical dilation, alligator forceps or other grasping instrument, or hysteroscopy may assist in removing an embedded Paragard.
- Make sure Paragard is intact upon removal.
- Removal may be associated with some pain and/or bleeding, vasovagal reactions (e.g. syncope, bradycardia), or seizure, especially in patients with a predisposition to these conditions.
3. Dosage Forms and Strengths
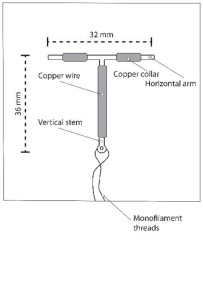
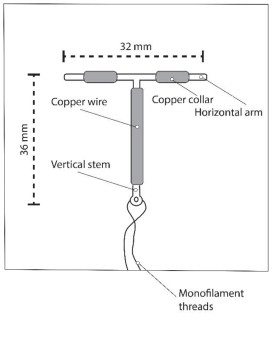
Paragard is a T-frame copper-containing intrauterine system (IUS) consisting of a polyethylene frame with barium sulfate measuring 32 mm horizontally and 36 mm vertically, with approximately 176 mg of copper wire wrapped around the vertical stem and an approximately 68.7 mg copper wire collar placed on each side of the horizontal arm with a total exposed copper surface area is 380 ± 23 mm², packaged with a sterile pre-assembled inserter as a single-use, disposable device in a tray. A monofilament polyethylene thread is tied through the tip of the vertical stem resulting in two white threads, each approximately 36.5 cm in length. Figure 1 displays the contents of the package [see Dosage and Administration (2.3)].
4. Contraindications
The use of Paragard is contraindicated when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy [see Warnings and Precautions (5.1, 5.2) and Use in Specific Populations (8.1)]
- Abnormalities of the uterus resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease (PID) [see Warnings and Precautions (5.4)]
- Postpartum endometritis or postabortal endometritis in the past 3 months [see Warnings and Precautions (5.4)]
- Known or suspected uterine or cervical malignancy
- Uterine bleeding of unknown etiology
- Untreated acute cervicitis or vaginitis or other lower genital tract infection
- Conditions associated with increased susceptibility to pelvic infections [see Warnings and Precautions (5.4)]
- Wilson's disease [see Warnings and Precautions (5.8)]
- A previously placed IUD or IUS that has not been removed
- Hypersensitivity to any component of Paragard including to copper or any of the trace elements present in the copper component of Paragard [see Adverse Reactions (6.2) and Description (11)]
5. Warnings and Precautions
5.1 Ectopic Pregnancy
Evaluate for possible ectopic pregnancy in any female who becomes pregnant while using Paragard because a pregnancy that occurs with Paragard in place is more likely to be ectopic than a pregnancy in the general population. However, because Paragard prevents most pregnancies, females who use Paragard have a lower risk of an ectopic pregnancy than sexually active females who do not use any contraception.
The incidence of ectopic pregnancy in the clinical trials with Paragard (which excluded females with a previous history of ectopic pregnancy) was approximately 0.06%. Ectopic pregnancy may require surgery and may result in loss of fertility.
5.2 Risks with Intrauterine Pregnancy
If intrauterine pregnancy occurs with Paragard in place and the strings are visible or can be retrieved from the cervical canal, remove Paragard because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Paragard may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Paragard, consider the following:
Septic Abortion
In females becoming pregnant with an intrauterine system (IUS), including Paragard in place, septic abortion, with septicemia, septic shock, and death, may occur [see Warnings and Precautions (5.3)]. Septic abortion typically requires hospitalization and treatment with intravenous antibiotics. Septic abortion may result in spontaneous abortion or a medical indication for pregnancy termination. A hysterectomy may be required if severe infection of the uterus occurs, which will result in permanent infertility.
Continuation of Pregnancy
If a female becomes pregnant with Paragard in place and if Paragard cannot be removed or the female chooses not to have it removed, warn her that failure to remove Paragard increases the risk of miscarriage, sepsis, premature labor, and premature delivery. Prenatal care should include counseling about these risks and that she should report immediately any flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leakage of fluid, or any other symptom that suggests complications of the pregnancy.
5.3 Sepsis
Severe infection or sepsis, including Group A streptococcal sepsis (GAS), have been reported following insertion of IUSs, including Paragard. In some cases, severe pain occurred within hours of insertion followed by sepsis within days. Because death from GAS is more likely if treatment is delayed, it is important to be aware of these rare but serious infections. Aseptic technique during insertion of Paragard is essential in order to minimize serious infections such as GAS [see Dosage and Administration (2.3)].
5.4 Pelvic Inflammatory Disease and Endometritis
Insertion of Paragard is contraindicated in the presence of known or suspected Pelvic Inflammatory Disease (PID) or endometritis [see Contraindications (4)]. IUSs, including Paragard, have been associated with an increased risk of PID, most likely due to organisms being introduced into the uterus during insertion. In the clinical trials with Paragard, the incidence of PID that resulted in the removal of Paragard was approximately 0.1% [see Clinical Studies (14)].
Counsel women who receive Paragard to notify a healthcare provider if they have complaints of lower abdominal or pelvic pain, odorous discharge, unexplained bleeding, fever, or genital lesions or sores. In such circumstances, perform a pelvic examination promptly to evaluate for possible pelvic infection.
Remove Paragard in cases of recurrent PID or endometritis, or if an acute pelvic infection is severe or does not respond to treatment.
PID can have serious consequences, such as tubal damage (leading to ectopic pregnancy or infertility), hysterectomy, sepsis, and death.
Females at Increased Risk for PID
PID or endometritis are often associated with a sexually transmitted infection (STI) and Paragard does not protect against STIs. The risk of PID or endometritis is greater for females who have multiple sexual partners, and also for females whose sexual partner(s) have multiple sexual partners. Females who have had PID or endometritis are at increased risk for a recurrence or re-infection. In particular, ascertain whether a female is at increased risk of infection (for example, leukemia, acquired immune deficiency syndrome (AIDS), intravenous drug abuse).
Asymptomatic PID
PID or endometritis may be asymptomatic but still result in tubal damage and its sequelae.
Treatment of PID or Endometritis in Patients Using Paragard
Remove Paragard in cases of recurrent endometritis or PID, or if an acute pelvic infection is severe or does not respond to treatment. Prophylactic antibiotics administered at the time of insertion do not appear to lower the incidence of PID.
Promptly assess and treat any female who develops signs or symptoms of PID. Perform appropriate testing for sexually transmitted infection and initiate antibiotic therapy promptly. Paragard does not need to be removed immediately. Reassess the patient in 48-72 hours. If no clinical improvement occurs, continue antibiotics and consider removal of Paragard. If the decision is to remove Paragard, start antibiotics prior to removal to avoid the potential risk for bacterial spread resulting from the removal procedure.
Actinomycosis
Actinomycosis has been associated with IUS use, including Paragard. Symptomatic women with known actinomycosis infection should have Paragard removed and receive antibiotics. Actinomycetes can be found in the genital tract cultures in healthy women without IUSs. The significance of actinomyces-like organisms on a Papanicolaou (PAP) smear in an asymptomatic IUS user is unknown, and this finding alone does not always require IUS removal and treatment. When possible, confirm a PAP smear diagnosis with cultures.
5.5 Embedment
Partial penetration or embedment of Paragard in the myometrium can make removal difficult. In some cases, surgical removal may be necessary. Breakage of an embedded Paragard during non-surgical removal has been reported [see Dosage and Administration (2.6)].
5.6 Perforation
Partial or total perforation of the uterine wall or cervix may occur during insertions, although the perforation may not be detected until sometime later. Perforation may reduce contraceptive efficacy and result in pregnancy. The incidence of perforation during or following Paragard insertion in clinical trials was 0.2% (13 out of 5344).
Delayed detection or removal of Paragard in cases of perforation may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal penetration, intestinal obstruction, abscesses and/or damage to adjacent organs.
A postmarketing safety study conducted in Europe (EURAS IUD) with IUSs, including copper IUSs, demonstrated an increased risk of perforation in lactating women. The risk of perforation may be increased if an IUS, such as Paragard, is inserted when the uterus is fixed, retroverted or not completely involuted during the postpartum period.
If perforation does occur, locate and remove Paragard promptly. Surgery may be required. Preoperative imaging followed by laparoscopy or laparotomy is often required to remove Paragard from the peritoneal cavity.
5.7 Expulsion
Partial or complete expulsion of Paragard has been reported, resulting in the loss of contraceptive protection. The incidence of expulsion in the clinical trials with Paragard was approximately 2.3%. Consider further diagnostic imaging, such as x-ray, to confirm expulsion if the IUS is not found in the uterus.
Paragard has been placed immediately after delivery, although the risk of expulsion may be increased when the uterus is not completely involuted at the time of insertion. Remove a partially expelled Paragard.
5.8 Wilson's Disease
Paragard may exacerbate Wilson's disease, a rare genetic disease affecting copper excretion; therefore, the use of Paragard is contraindicated in females of reproductive potential with Wilson's disease [see Contraindications (4)].
5.9 Bleeding Pattern Alterations
Paragard can alter the bleeding pattern and result in heavier and longer menstrual cycles with intermenstrual spotting.
In two clinical trials with Paragard, there were reports of oligomenorrhea and amenorrhea; however, a causal relationship between Paragard and these events could not be established. Menstrual changes were the most common medical reason for discontinuation of Paragard. Discontinuation rates for pain and bleeding combined were highest in the first year of use and diminished thereafter. The percentage of females who discontinued Paragard because of bleeding problems or pain during these studies ranged from 12% in the first year to 2% in Year 9. Females complaining of heavy vaginal bleeding should be evaluated and treated, and may need to discontinue Paragard [see Adverse Reactions (6.1)].
5.10 Magnetic Resonance Imaging (MRI) Safety Information
Non-clinical testing has demonstrated that Paragard is MR Conditional. A patient with Paragard can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 3.0 T or 1.5T
- Maximum spatial field gradient of 4,000 gauss/cm (40 T/m)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2 W/kg (Normal Operating Mode)
Under the scan conditions defined above, Paragard is expected to produce a maximum temperature rise of less than 0.5º C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the system extended less than 5 mm from the implant when imaged with a gradient echo pulse sequence and a 3.0 T MRI system.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Ectopic pregnancy [see Warnings and Precautions (5.1)]
- Intrauterine pregnancy [see Warnings and Precautions (5.2)]
- Septic abortion [see Warnings and Precautions (5.2)]
- Group A Streptococcal Sepsis (GAS) [see Warnings and Precautions (5.3)]
- Pelvic Inflammatory Disease and Endometritis [see Warnings and Precautions (5.4)]
- Embedment [see Warnings and Precautions (5.5)]
- Perforation [see Warnings and Precautions (5.6)]
- Expulsion [see Warnings and Precautions (5.7)]
- Bleeding Pattern Alterations [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure in two trials [see Clinical Studies (14)].
- The WHO Study 79914 was a randomized, multicenter, multinational study of copper T IUSs, including Paragard in 1,396 women outside the U.S. In the WHO Study, 100% were parous and the mean age at enrollment was 29 years old.
- The U.S. Composite Study was a meta-analysis that evaluated randomized, double-blind, comparative studies of copper T IUSs, including Paragard in 3,536 women in the U.S. In the U.S. Composite Study, 64% were nulliparous, 49% were nulligravida, 68% were under age 25 at the time of enrollment (median age 23 years old).
Table 2 shows discontinuation rates from the two clinical studies by adverse reaction and year.
|
*Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the U.S. Composite Study (3536 subjects) and the World Health Organization (1396 subjects) trials. |
||||||||||
| Year | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Number of Women at Start of Year |
4,932 |
3,149 |
2,018 |
1,121 |
872 |
621 |
563 |
483 |
423 |
325 |
| Expulsion | 5.7 | 2.5 | 1.6 | 1.2 | 0.3 | 0.0 | 0.6 | 1.7 | 0.2 | 0.4 |
| Bleeding/Pain | 11.9 | 9.8 | 7.0 | 3.5 | 3.7 | 2.7 | 3.0 | 2.5 | 2.2 | 3.7 |
| Other Medical Event | 2.5 | 2.1 | 1.6 | 1.7 | 0.1 | 0.3 | 1.0 | 0.4 | 0.7 | 0.3 |
The following adverse reactions have also been observed: anemia, backache, dysmenorrhea, dyspareunia, complete or partial expulsion, prolonged menstrual flow, menstrual spotting, pain and cramping, and vaginitis.
Study CSIPD-001
The Paragard inserter that enables single-hand insertion was evaluated in Study CSIPD-001. A total of 117 females of reproductive potential aged 18 to 49 years, underwent Paragard insertion and were followed for up to 12 weeks of Paragard use. Subjects were predominantly white (76%), 45% were parous, and 35% were obese. Successful placement of Paragard with first attempt occurred in 91% of the subjects and 99% with two insertion attempts. Adverse reactions of special interest occurring during the study were IUS expulsion (2.6%), vasovagal reaction (2.6%), IUS malposition (1.7%), partial uterine perforation (0.9%), and IUS embedment (0.9%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Paragard. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: abdominal distension, nausea
General Disorders and Administration Site Conditions: device breakage, pyrexia; copper wire breakage
Immune System Disorders: allergy to metals, hypersensitivity
Infections and Infestations: endometritis/uterine infection
Musculoskeletal and Connective Tissue Disorders: muscle spasms
Nervous System Disorders: dizziness
Reproductive System and Breast Disorders: amenorrhea
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
7. Drug Interactions
No drug-drug interaction or drug-herbal supplement interaction studies have been conducted with Paragard.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Use of Paragard is contraindicated for use in pregnant females because there is no need for pregnancy prevention in a female who is already pregnant and Paragard may cause adverse pregnancy outcomes. If a female becomes pregnant with Paragard in place, there is an increased risk of miscarriage, sepsis, premature labor, and premature delivery [see Contraindications (4) and Warnings and Precautions (5.1, 5.2)]. Advise the female of the potential risks if pregnancy occurs with Paragard in place.
Published studies on pregnancy outcomes exposed to copper IUSs report up to 27% miscarriage when the IUS was removed compared to 77% miscarriage when the IUS remained in the uterus. Studies on Paragard and birth defects have not been conducted.
8.2 Lactation
Risk Summary
No difference has been detected in concentration of copper in human milk before and after insertion of copper IUSs, including Paragard. There is no information on the effect of copper in a breastfed child or the effect on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Paragard and any potential adverse effects on the breastfed child from Paragard.
11. Paragard Description
11.1 Paragard IUS
Paragard (intrauterine copper contraceptive) is a T-shaped intrauterine system (IUS), measuring 32 mm horizontally and 36 mm vertically, with a 3 mm diameter bulb at the tip of the vertical stem. (See Figure 10) [see Dosage and Administration (2.3)]. A monofilament polyethylene thread is tied through the tip, resulting in two white threads, each approximately 36.5 cm in length, to aid in detection and removal of the intrauterine system. The T-frame is made of polyethylene with barium sulfate to aid in detecting the intrauterine system under x-ray. Paragard also contains copper (approximately 176 mg of wire wrapped around the vertical stem and an approximately 68.7 mg collar placed on each side of the horizontal arm). The total exposed copper surface area is 380 ± 23 mm². One Paragard weighs less than one (1) gram.
Figure 10 – Paragard IUS
Paragard, its components, and the packaging are not made with natural rubber latex.
11.2 Inserter
Paragard is packaged with a sterile pre-assembled inserter as a single-use, disposable device in a tray and in a Tyvek® polyethylene pouch. A moveable blue flange on the insertion tube aids in gauging the depth of insertion through the cervical canal and into the uterine cavity (See Figure 11) [see Dosage and Administration (2.3)].
Figure 11 – Paragard with Inserter
12. Paragard - Clinical Pharmacology
12.1 Mechanism of Action
Copper continuously released into the uterine cavity contributes to the contraceptive effectiveness of Paragard. Mechanism(s) by which copper enhances contraceptive efficacy include interference with sperm transport and fertilization of an egg, and possibly prevention of implantation.
14. Clinical Studies
The efficacy of Paragard for use in females of reproductive potential for prevention of pregnancy for up to 10 years was demonstrated in two trials:
- WHO Study 79914 was a randomized, multicenter, multinational study of copper T IUSs, including Paragard in 1,396 women outside the U.S. In the WHO study, 100% were parous and the mean age at enrollment was 29 years old.
- The U.S. Composite Study was a meta-analysis that evaluated several randomized, double-blind, comparative studies of copper T IUSs, including the Paragard in 3,536 women in the U.S. In the U.S. study, 64% were nulliparous, 49% were nulligravida; 68% were under age 25 at the time of enrollment (median age 23 years old).
The pregnancy rate in the two clinical studies with Paragard was less than 1 pregnancy per 100 women each year (see Table 3).
|
*Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the U.S. Composite Study (3536 subjects) and the World Health Organization (1396 subjects) trials. |
||||||||||
| Year | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Pregnancy | 0.7 | 0.3 | 0.6 | 0.2 | 0.3 | 0.2 | 0.0 | 0.4 | 0.0 | 0.0 |
| Number of Women at Start of Year |
4,932 |
3,149 |
2,018 |
1,121 |
872 |
621 |
563 |
483 |
423 |
325 |
16. How is Paragard supplied
Paragard (intrauterine copper contraceptive) is available in cartons of 1 (one) sterile unit (NDC 59365-5129-1).
Each Paragard is white, T-shaped, and measures 32 mm horizontally and 36 mm vertically, with approximately 176 mg of copper wire wrapped around the vertical stem and an approximately 68.7 mg copper wire collar placed on each side of the horizontal arms, and with a monofilament polyethylene thread tied through the tip of the vertical stem [see Dosage and Administration (2.3)]. The T-frame is made of polyethylene with barium sulfate. Each Paragard is packaged with a sterile pre-assembled inserter as a single-use, disposable device in a tray and in a Tyvek® polyethylene pouch.
Store at controlled room temperature: 59° to 86°F (15° to 30°C).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information). Before inserting Paragard, counsel patients on the following:
Sexually Transmitted Infections: Advise patients that Paragard does not protect against HIV infection and other sexually transmitted diseases.
Ectopic Pregnancy: Advise patients to report pregnancies and be evaluated immediately, as a pregnancy with Paragard in place is more likely to be an ectopic pregnancy, and the risks of ectopic pregnancy include the loss of fertility [see Warnings and Precautions (5.1)].
Intrauterine Pregnancy: Advise patients to report pregnancies and be evaluated immediately, as an intrauterine pregnancy with Paragard in place, may result in septic abortion, with septicemia, septic shock, and possible death. Septic abortion typically requires hospitalization and treatment with intravenous antibiotics. Septic abortion may result in spontaneous abortion or a medical indication for pregnancy termination. A hysterectomy may be required if severe infection of the uterus occurs, which will result in permanent infertility. If Paragard remains in the uterus during a pregnancy, there is an increased risk of miscarriage, sepsis, premature labor and premature delivery. Advise patients that a pregnancy must be followed closely and advise patients to report immediately any symptom, such as flu- like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leaking of fluid, or any other symptom that suggests complications of the pregnancy [see Warnings and Precautions (5.2)].
Sepsis: Advise patients that sepsis may occur after insertion of Paragard and to seek immediate treatment for fever and pelvic pain that occurs soon after insertion, as untreated sepsis can result in death [see Warnings and Precautions (5.3)].
Pelvic Inflammatory Disease: Advise patients that pelvic inflammatory disease (PID) may occur after insertion of Paragard and that PID can cause tubal damage leading to ectopic pregnancy or infertility, or can necessitate hysterectomy, or cause death. Advise patients to recognize and report promptly any symptoms of PID including development of menstrual disorders (prolonged or heavy bleeding), unusual vaginal discharge, abdominal or pelvic pain or tenderness, dyspareunia, chills, and fever [see Warnings and Precautions (5.4)].
Embedment, Perforation, and Expulsion: Advise patients regarding the risks of embedment, perforation, and expulsion and inform patients that in some cases, removal of Paragard may be difficult and surgical removal may be necessary. Inform patients that perforation can cause infection, scarring, damage to other organs, pain, or infertility. Inform patients that excessive pain or vaginal bleeding during IUS placement, worsening pain or bleeding after placement, or the inability to feel the IUS strings may occur with IUS perforation and expulsion. There is no protection from pregnancy if Paragard is displaced or expelled [see Warnings and Precautions (5.5, 5.6, 5.7).].
Bleeding Pattern Alterations: Advise patients that heavier or longer periods and spotting between periods may occur. Instruct patients to report continued or severe symptoms to their healthcare provider [see Warnings and Precautions (5.9)].
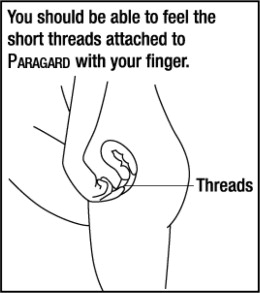
Checking for Paragard: Advise patients to check that Paragard is still in the uterus by reaching up to the top of the vagina with clean fingers to feel the two threads. Inform patients not to pull on the threads, which could displace Paragard. Advise patients to promptly report if there are changes in the length of the two threads, if they cannot locate the threads, or they can feel any other part of Paragard besides the threads.
Magnetic Resonance Imaging (MRI) Safety Information: Inform patients that Paragard can be safely scanned with MRI only under specific conditions. Instruct patients who will have an MRI to tell their healthcare provider that they have Paragard [see Warnings and Precautions (5.10)].
Medical Diathermy: Instruct patients to tell their healthcare provider that they have Paragard prior to undergoing medical diathermy [see Warnings and Precautions (5.11)].
Other Important Information:
- Advise patients to contact their healthcare provider if any of the following occur:
- Pelvic pain or pain during sex
- Unusual vaginal discharge or genital sores
- Possible exposure to STIs
- HIV positive seroconversion in herself or her partner
- Severe or prolonged vaginal bleeding
- Missed period
- Advise patients that they may use tampons or pads while wearing Paragard.
Manufactured by:
CooperSurgical, Inc.
Trumbull, CT 06611
|
This Patient Information has been approved by the U.S. Food and Drug Administration |
||
| PATIENT INFORMATION
PARAGARD® ('par-uh-gahrd) (intrauterine copper contraceptive) |
||
| Paragard does not protect against HIV infection (AIDS) or other sexually transmitted infections (STIs).
Read this Patient Information carefully before you decide if Paragard is right for you. This information does not take the place of talking with your gynecologist or other healthcare provider who specializes in women's health. If you have any questions about Paragard, ask your healthcare provider. You should also learn about other birth control methods to choose the one that is best for you. |
||
What is Paragard?
|
||
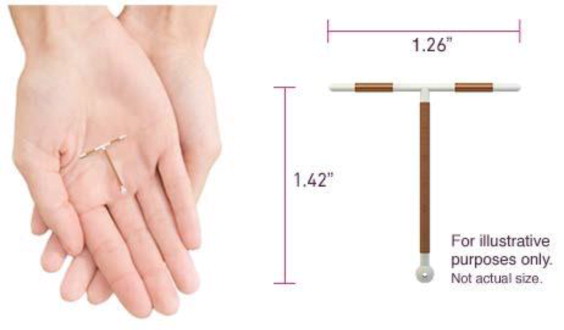 |
||
| What if I need birth control for more than 10 years?
Paragard must be removed on or before 10 years from the date of insertion. Your healthcare provider can place a new Paragard during the same office visit if you choose to continue using Paragard. |
||
| What if I want to stop using Paragard?
Paragard is intended for use up to 10 years, but you can stop using Paragard at any time by asking your healthcare provider to remove it. You could become pregnant as soon as Paragard is removed; however, if you do not want to become pregnant you should use another method of birth control. Talk to your healthcare provider about the best birth control methods for you. |
||
| What if I change my mind about birth control and want to become pregnant in less than 10 years?
Your healthcare provider can remove Paragard at any time before the 10 years after placement. You may become pregnant as soon as Paragard is removed. |
||
| How does Paragard work?
Paragard works by preventing sperm from reaching the egg, preventing sperm from fertilizing the egg, or possibly preventing attachment (implantation) in the uterus. Paragard does not stop your ovaries from making an egg (ovulating) each month. |
||
| How well does Paragard work for contraception?
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant. Paragard, an intrauterine system (IUS), is in the box at the top of the chart. |
||
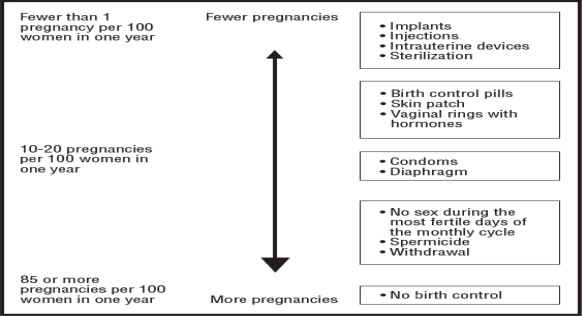 |
||
| Who might use Paragard?
You might choose Paragard if you:
|
||
Do not use Paragard if you:
|
||
Before having Paragard placed, tell your healthcare provider if you have:
|
||
| How is Paragard placed?
Paragard is placed in your uterus during an in-office visit. First, your healthcare provider will examine your pelvis to find the exact position of your uterus. Your healthcare provider will then cleanse your vagina and cervix with an antiseptic solution and then, measure your uterus. Your healthcare provider will then slide a plastic tube containing Paragard into your uterus. The tube is removed, leaving Paragard inside your uterus. Two white threads will extend into your vagina. The threads are trimmed so they are just long enough for you to feel with your fingers when doing a self-check. As Paragard goes in, you may feel cramping or pinching. You may have some bleeding. Some women feel faint, nauseated, or dizzy for a few minutes afterwards. Your healthcare provider may ask you to lie down until you are feeling better, and to get up slowly. |
||
| Should I check that Paragard is in place?
Yes, you should check that Paragard is in proper position by feeling the threads. It is a good habit to do this 1 time a month. Your healthcare provider should teach you how to check that Paragard is in place. First, wash your hands with soap and water. You can check by reaching up to the top of your vagina with clean fingers to feel the 2 threads. Do not pull on the threads. |
||
| If you feel changes in the length of the 2 threads, you cannot feel the threads, or you can feel any other part of Paragard other than the threads, Paragard may not be in the right position and may not prevent pregnancy. Use back-up birth control (such as condoms or spermicide) and ask your healthcare provider to check that Paragard is still in the right place. If Paragard is accidentally removed, you may be at risk of pregnancy, and should talk to a healthcare provider. |
||
| How soon after placement of Paragard should I return to my healthcare provider?
Call your healthcare provider if you have any questions or concerns (see “When should I call my healthcare provider?”). Otherwise you should return to your healthcare provider for a follow-up visit after your first menses after Paragard is placed to make sure that Paragard is in the right position. |
||
|
What if I become pregnant while using Paragard?
|
||
| How will Paragard change my periods?
Your period may become heavier and longer. You may also have frequent spotting between periods. |
||
| Is it safe to breastfeed while using Paragard?
You may use Paragard when you are breastfeeding. The risk of Paragard becoming attached to (embedded) or going through the wall of the uterus is increased if Paragard is placed while you are breastfeeding. |
||
| Will Paragard interfere with sexual intercourse?
You and your partner should not feel Paragard during intercourse. Paragard is placed in the uterus, not in the vagina. Sometimes your partner may feel the threads. If this occurs, or if you or your partner experience pain during sex, talk with your healthcare provider. |
||
| Can I have an MRI with Paragard in place?
Paragard can be safely scanned with MRI only under specific conditions. Before you have an MRI, tell your healthcare provider that you have Paragard, an intrauterine device (IUD), in place. |
||
| Before you have a medical procedure using heat therapy tell your healthcare provider that you have Paragard in place. | ||
What are the possible side effects of Paragard? Paragard can cause serious side effects, including:
|
||
| Common side effects of Paragard include: | ||
|
|
|
| This is not a complete list of possible side effects with Paragard. For more information, ask your healthcare provider. Tell your healthcare provider about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088. |
||
|
After Paragard has been placed, when should I call my healthcare provider?
|
||
| General information about the safe and effective use of Paragard.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider for information about Paragard that is written for health professionals. |
||
| Paragard and its components are not made with natural rubber latex. | ||
| Paragard® is a registered trademark of CooperSurgical, Inc. The other brands listed are trademarks of their respective owners. Manufactured by: CooperSurgical, Inc. Trumbull, CT 06611 For more information, call CooperSurgical, Inc. at 1-877-727-2427. |
||
Principal Display Panel – Carton Label
Cooper Surgical®
Paragard® T380A
intrauterine copper contraceptive
1 UNIT
Each unit wound with approximately 176 mg of copper wire. In addition, a single copper sleeve is swaged on each of the two transverse arms. Each sleeve contains approximately 68.7 mg of copper. The total surface area of copper on the device is 380 ± 23mm2.
IMPORTANT: Read the Preparation Instructions and Insertion Procedure before initiating Paragard insertion. To be inserted in the uterus only by a licensed clinician or under the supervision of a physician. See detailed instructions in the Prescribing Information.
NOTE: Patient Information is provided with each unit. Please ensure the Patient Information is provided to and reviewed with each patient before insertion. Store at controlled room temperature: 59º to 86ºF (15° to 30°C).
CooperSurgical, Inc. | 95 Corporate Drive | Trumbull, CT 06611 USA
RX ONLY
NDC 59365-5129-1
| PARAGARD T 380A
copper intrauterine device |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CooperSurgical, Inc. (801895244) |
More about ParaGard (copper topical)
- Compare alternatives
- Pricing & coupons
- Reviews (1,306)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous vaginal agents