DermOtic Oil Prescribing Information
Package insert / product label
Generic name: fluocinolone acetonide
Dosage form: ear drops
Drug class: Otic steroids
Medically reviewed by Drugs.com. Last updated on Sep 17, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
DERMOTIC OIL (fluocinolone acetonide) ear drops, for otic use
Initial U.S. Approval: 1988
Indications and Usage for DermOtic Oil
DERMOTIC® OIL is a corticosteroid indicated for the topical treatment of chronic eczematous external otitis in adults and pediatric patients 2 years of age and older. (1)
DermOtic Oil Dosage and Administration
Dosage Forms and Strengths
Ear drops, containing 0.01% fluocinolone acetonide, supplied in bottles containing 20 mL. (3)
Contraindications
None. (4)
Warnings and Precautions
-
Endocrine System Adverse Reactions:
o Topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing’s syndrome, hyperglycemia, and glucosuria. (5.1)
o Pediatric patients may be more susceptible to systemic toxicity from equivalent doses. (5.1,8.4)
o Systemic absorption may require evaluation for HPA axis suppression. Potent corticosteroids use on large areas, prolonged use or occlusive use, altered skin barrier, liver failure, and young age may increase systemic absorption. Modify use should HPA axis suppression develop. (5.1). - Local Adverse Reactions: Local adverse reactions may include atrophy, striae irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis, and may be more likely with occlusive use or more potent corticosteroids. (5.2, 6.1)
- Ophthalmic Adverse Reactions: May increase the risks of glaucoma and posterior subcapsular cataract. Avoid contact of DERMOTIC OIL with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation. (5.3)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (≥ 1%) were headache (3%), URI (2%), cough (2%), eczematous otitis (1%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hill Dermaceuticals, Inc. at 1-800-344-5707 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
Related/similar drugs
prednisone, Dupixent, Temovate, Lidex, Clobex, Olux
Full Prescribing Information
2. DermOtic Oil Dosage and Administration
DERMOTIC OIL is for otic administration only. Not for oral, ophthalmic, or intravaginal use.
Apply DERMOTIC OIL into the affected ear using the supplied ear dropper. To apply, tilt head to one side so that the ear is facing up. Then gently pull the ear lobe backward and upward and apply 5 drops of DERMOTIC OIL into the ear. Keep head tilted for about a minute to allow DERMOTIC OIL to penetrate lower into the ear canal. Gently pat excess material dripping out of the ear using a clean cotton ball. Follow these instructions twice each day for 7 to 14 days.
Discontinue DERMOTIC OIL when control of disease is achieved within 2 weeks, or contact the healthcare provider if no improvement is seen within 2 weeks.
Do not use on the face, axillae, or groin unless directed by the healthcare provider. Do not apply to intertriginous areas due to the increased risk of local adverse reactions [see Adverse Reactions (6) and Use in Specific Populations (8.4)].
3. Dosage Forms and Strengths
Ear drops, containing 0.01% fluocinolone acetonide supplied in bottles containing 20 mL (dropper included).
5. Warnings and Precautions
5.1 Endocrine System Adverse Reactions
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency. Cushing’s syndrome, hyperglycemia, and glucosuria can result from systemic absorption of topical corticosteroids.
HPA axis suppression and Cushing’s syndrome have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and subnormal response to ACTH stimulation. Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios [see Use in Specific Populations (8.4)].
Conditions which increase systemic absorption include the use of more potent corticosteroids, use over large surface areas, use over prolonged periods, use of occlusive dressings, altered skin barrier, liver failure, and young age. Use of more than one corticosteroid-containing product at the same time may increase total systemic corticosteroid exposure.
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. The ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression.
If HPA axis suppression is documented, an attempt should be made to withdraw the drug to reduce the frequency of application, or to substitute a less potent corticosteroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids.
5.2 Local Adverse Reactions
Local adverse reactions may occur with use of topical corticosteroids, including DERMOTIC OIL, and may be more likely to occur with occlusive use, prolonged use, or use of higher potency corticosteroids. Some local adverse reactions may be irreversible. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria [see Adverse Reactions (6.1)].
5.3 Ophthalmic Adverse Reactions
Use of topical corticosteroids may increase the risks of glaucoma and posterior subcapsular cataract. Glaucoma and cataracts have been reported in postmarketing experience with the use of topical corticosteroid products. Avoid contact of DERMOTIC OIL with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
5.4 Allergic Contact Dermatitis
Use of topical corticosteroids can cause allergic contact dermatitis. Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed by patch testing.
5.5 Concomitant Skin Infections
Use of topical corticosteroids may delay healing or worsen concomitant skin infections. Treat concomitant skin infections with an appropriate antimicrobial agent. If the infection persists unchanged, discontinue DERMOTIC OIL until the infection has been adequately treated.
5.6 Use in Peanut Sensitive Individuals
Use caution in prescribing DERMOTIC OIL for peanut sensitive individuals [see Description (11)].
Should signs of hypersensitivity present (wheal and flare reactions, pruritus, or other manifestations), or should disease exacerbations occur, discontinue DERMOTIC OIL immediately and institute appropriate therapy.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
• Endocrine System Adverse Reactions [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)]
• Local Adverse Reactions [see Warnings and Precautions (5.2)]
• Ophthalmic Adverse Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In trials that enrolled 154 subjects (adults and pediatric subjects 2 years and older) with chronic eczematous external otitis who were treated with five drops per ear of DERMOTIC OIL twice daily for a maximum 14 days of treatment, the following adverse reactions were reported:
| Adverse Reaction | n (%) |
|---|---|
| Headache | 4 (3) |
| URI | 3 (2) |
| Cough | 3 (2) |
| Eczematous otitis | 2 (1) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of products containing topical corticosteroids. Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Endocrine Disorders: HPA axis suppression and Cushing’s syndrome [see Use in Specific Populations (8.4)]
- Eye Disorders: glaucoma and cataracts [see Warnings and Precautions (5.3)]
- Nervous System Disorders: intracranial hypertension including bulging fontanelles, headaches, and bilateral papilledema [see Use in Specific Populations (8.4)]
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from case reports, case series, and observational studies on fluocinolone acetonide use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Observational studies suggest maternal use of high to super-high potency topical steroids may be associated with an increased risk of low birthweight infants. Advise pregnant women to use DERMOTIC OIL on the smallest area of skin and for the shortest duration possible.
Corticosteroids can cause fetal malformations in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids cause fetal malformations after dermal application in laboratory animals.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of fluocinolone acetonide in breast milk or its effects on the breastfed infant or on milk production. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DERMOTIC OIL and any potential adverse effects on the breastfed infant from DERMOTIC OIL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of DERMOTIC OIL for the topical treatment of chronic eczematous external otitis have been established in pediatric patients aged 2 years and older.
Safety and effectiveness of DERMOTIC OIL in pediatric patients with chronic eczematous external otitis below the age of 2 years have not been established.
Systemic Adverse Reactions in Pediatric Patients
HPA Axis suppression, Cushing’s syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and subnormal response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk for systemic adverse reactions than are adults when treated with topical corticosteroids [see Warnings and Precautions (5.1)].
Evaluation in Peanut-Sensitive Pediatric Patients
A clinical trial was conducted to assess the safety of the formulation of DERMOTIC OIL, which contains refined peanut oil, in patients with known peanut allergies. The trial enrolled 13 pediatric subjects with atopic dermatitis, 6 to 17 years of age. DERMOTIC OIL is not approved for the treatment of atopic dermatitis. Of the 13 subjects, 9 were Radioallergosorbent Test (RAST) positive to peanuts and 4 had no peanut sensitivity (controls). The trial evaluated the subjects’ responses to both prick test and patch test utilizing refined peanut oil, the formulation of DERMOTIC OIL and histamine/saline controls. Subjects were also treated with the formulation of DERMOTIC OIL twice daily for 7 days. Prick test and patch test results for all 13 subjects were negative to the formulation of DERMOTIC OIL and the refined peanut oil. One of the 9 peanut-sensitive subjects experienced an exacerbation of atopic dermatitis after 5 days of use on the formulation of DERMOTIC OIL.
Evaluation in Pediatric Patients 2 to 6 years old
Use of the formulation of DERMOTIC OIL in pediatric patients 2 to 6 years old is supported by open-label safety trials conducted in 33 pediatric subjects (20 subjects ages 2 to 6 years, 13 subjects ages 7 to 12 years) with moderate to severe stable atopic dermatitis. Baseline body surface area involvement was 50% to 75% in 15 subjects and greater than 75% in 18 subjects. Subjects were treated with the formulation of DERMOTIC OIL twice daily for 4 weeks. Morning pre-stimulation cortisol and post-ACTH stimulation cortisol levels were obtained in each subject the beginning of the trial and at the end of 4 weeks of treatment. At the end of treatment, 4 out of 18 subjects aged 2 to 5 years showed low pre-stimulation cortisol levels (3.2 to 6.6 µg/dL; normal: cortisol > 7µg/dL) but all had normal responses to 0.25 mg of ACTH stimulation (cortisol > 18 µg/dL) [see Clinical Pharmacology (12.2)].
11. DermOtic Oil Description
DERMOTIC OIL (fluocinolone acetonide) 0.01% ear drops contains fluocinolone acetonide [(6α, 11β, 16α)-6,9-difluoro-11,21-dihydroxy-16, 17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene3,20-dione, cyclic 16,17 acetal with acetone], a synthetic corticosteroid for topical dermatologic use. Chemically, fluocinolone acetonide is C24H30F2O6. It has the following structural formula:
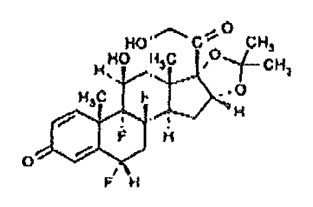
Fluocinolone acetonide has a molecular weight of 452.50. It is a white crystalline powder that is odorless, stable in light, and melts at 270°C with decomposition; soluble in alcohol, acetone and methanol; slightly soluble in chloroform; insoluble in water.
Each gram of DERMOTIC OIL contains approximately 0.11 mg of fluocinolone acetonide in a blend of oils, which contains isopropyl alcohol, isopropyl myristate, light mineral oil, oleth-2, refined peanut oil and fragrances.
DERMOTIC OIL is formulated with 48% refined peanut oil. The bulk refined peanut oil, used in DERMOTIC OIL is heated at 246°C (475°F) for at least 15 minutes. The refined peanut oil used in DERMOTIC OIL is routinely tested for peanut proteins through amino acid analysis; the quantity of amino acids is below 0.5 parts per million (ppm).
12. DermOtic Oil - Clinical Pharmacology
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in eczematous external otitis is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay
DERMOTIC OIL is in the low to medium range of potency as compared with other topical corticosteroids in vasoconstrictor studies. However, similar blanching scores do not necessarily imply therapeutic equivalence.
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
HPA axis suppression was evaluated in 33 pediatric subjects 2 to 12 years old (20 subjects ages 2 to 6 years, 13 subjects ages 7 to 12 years) with moderate to severe stable atopic dermatitis. Subjects were treated with the formulation of DERMOTIC OIL twice daily for 4 weeks. Baseline body surface area involvement was 50% to 75% in 15 subjects and greater than 75% in 18 subjects. Morning pre-stimulation cortisol and post-ACTH stimulation cortisol levels were obtained in each subject at the beginning of the trial and at the end of 4 weeks of treatment. At the end of treatment, 4 out of 18 subjects aged 2 to 5 years showed low pre-stimulation cortisol levels (3.2 to 6.6 µg/dL; normal cortisol >7 µg/dL) but all had normal responses to 0.25 mg of ACTH stimulation (cortisol > 18 µg/dL) [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the product formulation and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may increase percutaneous absorption. The use of pharmacodynamic endpoints for assessing the systemic exposure of topical corticosteroids may be necessary due to the fact that circulating levels are often below the level of detection. Once absorbed through the skin, topical corticosteroids are metabolized, primarily in the liver, and are then excreted by the kidneys. Some corticosteroids and their metabolites are also excreted in the bile.
13. Nonclinical Toxicology
13.1 Carcinogenesis, mutagenesis, impairment of fertility
No carcinogenicity, genotoxicity, or fertility studies were conducted with DERMOTIC OIL. However, some corticosteroids are genotoxic in various genotoxicity tests (i.e., the in vitro human peripheral blood lymphocyte chromosome aberration assay with metabolic activation, the in vivo mouse bone marrow micronucleus assay, the Chinese hamster micronucleus test and the in vitro mouse lymphoma gene mutation assay).
14. Clinical Studies
In two vehicle-controlled trials (Trial 1 and Trial 2), 154 subjects (adults and pediatric subjects 2 years of age and older) with chronic eczematous external otitis were treated with 5 drops per ear of DERMOTIC OIL twice daily for 7 days. Efficacy was assessed on Day 7 by clearance of the signs and symptoms of eczematous external otitis, and the results are presented in the following table:
16. How is DermOtic Oil supplied
DERMOTIC OIL (fluocinolone acetonide oil) 0.01% ear drops is supplied in bottles containing 20 mL, (dropper included) (NDC # 68791-103-20).
17. Patient Counseling Information
Administration Instructions
Advise patients that DERMOTIC OIL is for otic administration only and not for oral, ophthalmic, or intravaginal use [see Dosage and Administration (2)].
Advise patients to avoid use of DERMOTIC OIL on the face, axillae, or groin unless directed by their healthcare provider [see Dosage and Administration (2)].
Advise patients to discontinue therapy when control of disease is achieved. Instruct patients to contact their healthcare provider if no improvement is seen within 2 weeks [see Dosage and Administration (2)].
Endocrine System Adverse Reactions
Instruct patients not to use other corticosteroid-containing products while using DERMOTIC OIL without first consulting their healthcare provider [see Warnings and Precautions (5.1)].
Ophthalmic Adverse Reactions
Advise patients to avoid contact with the eyes and in case of contact, wash eyes liberally with water. Instruct patients to tell their healthcare provider if they develop any visual symptoms [see Warnings and Precautions (5.3)].
Pregnancy and Lactation
Advise patients to use DERMOTIC OIL on the smallest area of skin and for the shortest duration possible while pregnant or breastfeeding. Advise patients that are breastfeeding not to apply DERMOTIC OIL directly to the nipple and areola to avoid direct infant exposure [See Use in Specific Populations (8.1 and 8.2)].
| DERMOTIC
fluocinolone acetonide oil |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Royal Pharmaceuticals (078374385) |
| Registrant - Royal Pharmaceuticals (078374385) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HILL DERMACEUTICALS, INC. | 098366990 | MANUFACTURE(68791-103) | |
More about DermOtic Oil (fluocinolone otic)
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- Generic availability
- Drug class: otic steroids
- Breastfeeding
- En español

