Anticholium Injection Prescribing Information
Package insert / product label
Generic name: physostigmine salicylate
Dosage form: injection
Drug class: Antidotes
Medically reviewed by Drugs.com. Last updated on Oct 10, 2023.
On This Page
HEALTH CARE PROVIDER LETTER
September 2023
IMPORTANT PRESCRIBING INFORMATION
|
Subject: Temporary Importation of Anticholium® (physostigmine salicylate) Ampules with Foreign, non-U.S. Labeling to Address Supply Shortage |
Dear Healthcare Provider,
To address the ongoing shortages of physostigmine salicylate injection in the United States, Dr. Franz Köhler Chemie GmbH (Koehler) in conjunction with Provepharm Inc. (Provepharm) and Direct Success, Inc. (Direct Success) is coordinating with the U.S. Food and Drug Administration (FDA) to temporarily import Anticholium® (Physostigmine salicylate, 2mg/5ml, injection solution for intravenous use) into the U.S. market. Koehler’s Anticholium® (Physostigmine salicylate, 2mg/5ml, injection solution for intravenous use), marketed and manufactured in Germany for Koehler, is not FDA-approved.
At this time, no other entity except Provepharm or its distributor Direct Success is authorized by the FDA to import or distribute physostigmine salicylate injection in the U.S.
Effective immediately, Provepharm will distribute the following presentations of Anticholium® (physostigmine salicylate) to address the critical shortage:
|
Product Description |
Strength |
Packaging |
NDC number |
Lot/ Batch # |
Expiration Date (Labeled) |
|
Anticholium® (physostigmine salicylate) |
2 mg/5 mL (0.4 mg/mL) injection for intravenous use |
5 x 5 mL ampules |
81284-831-05 |
2315751 |
2026-05-31 |
To place an order, please contact Direct Success at Distribution@DSuccess.com or 1-877-404-3338.
The U.S. imported Anticholium (physostigmine salicylate) injection contains nitrogen even though it is not listed on the enclosed Instructions for Use (IFU).
The barcode on the imported product label may not register accurately on the U.S. scanning systems. Institutions should manually input the imported product information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
In addition, the packaging of the imported product does not include serialization information. Koehler’s Anticholium® does not meet the Drug Supply Chain Security Act (DSCSA) requirements for the Interoperable Exchange of Information for Tracing of Human, Finished Prescription Drugs.
There are key differences between the U.S. marketed physostigmine salicylate injection and ANTICHOLIUM® noted in Table 1 below.
|
Table 1. Key Differences between physostigmine salicylate injection and Anticholium® |
||
|
Product Names |
U.S. physostigmine salicylate injection |
Anticholium® (German imported product) |
|
Indication |
To reverse the effect upon the central nervous system, caused by clinical or toxic dosages of drugs capable of producing the anticholinergic syndrome. |
Indicated for central anticholinergic syndrome (CAS), delayed postoperative awakening and shivering Indicated as an antidote and/or antagonist in case of intoxication with and/or overdose of: alcohol, tropane alkaloids, amanita pantherina and amanita muscaria,tricyclic antidepressants, antiemetics/antihistamines, neuroleptic drugs, benzodiazepines, antispasmodics, parkinson disease medication, baclofen, 4-hydroxybutyric acid (GHB), inhalation anesthetics, ketamine, 3-quinuclidinyl benzilate |
|
Administration |
Injection for intramuscular or intravenous use |
Injection solution for intravenous use only |
|
Packaging |
2 mL in 1 AMPULE |
5 ampules of 5 ml containing 2 mg of physostigmine salicylate |
When using Anticholium® (Physostigmine) from Koehler the following advice has to be taken into account:
Dosing and administration:
For the treatment of postoperative awakening disorders in adults:
Slowly inject physostigmine intravenously at a dose of 0.04 mg/kg body weight (approx. 1 mg/min), the maximum individual dose is 2 mg. In case of insufficient effect, give subsequent injections after 5 to 20 minutes at the earliest, after a positive evaluation of the effect of the first injection.
Treatment in cases of intoxication:
Adults: Slowly inject 0.04 mg/kg body weight (2 mg) of physostigmine salicylate intravenously and subsequently inject 1-4 mg every 20 minutes. Repeat the effective dose if the intoxication symptoms recur, also in the form of a continuous infusion if deemed useful.
Children and adolescents: Infants: Start with a low dose of 0.5 mg of physostigmine salicylate administered intravenously, repeat this dose every 5 minutes up to the overall dose of 2 mg, as long as the toxic, anticholinergic symptoms continue to persist and no cholinergic symptoms occur.
Method of administration:
Anticholium® should be administered intravenously slowly or as brief infusion in 50 ml physiological saline solution over 10-15 minutes. A general criterion for adequate physostigmine dosing is the recognizable recovery of mental abilities and responsiveness (e.g. specifying name, address, date).
For further Information please refer to the full prescribing information enclosed.
Prescription and Labeling:
Anticholium® will be available only by prescription in the U.S. However, the imported product does not have the statement “Rx only” on the labeling.
There is no barcode on this product for use with U.S. barcode scanning systems. Alternative procedures should be followed to ensure that the correct drug product is being used and administered to individual patients.
Reporting Adverse Events:
Healthcare providers should report adverse events associated with the use of Koehler’s Anticholium® to Provepharm at:
Phone: 1-833-727-6556
email: safety-us@provepharm.com
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form https://www.accessdata.fda.gov/scripts/medwatch/index.cfm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
You may also contact our medical information department at medicalaffairs@provepharm.com if you have any questions about the information contained in this letter or the safe and effective use of Anticholium®.
This letter is not intended as a complete description of the benefits and risks related to the use of Anticholium®. Please refer to the enclosed full prescribing information.
For additional information, please contact Dr. Franz Köhler Chemie GmbH at info@koehler-chemie.de or visit www.koehler-chemie.de
Finally, please ensure that your staff and others in your institution who may be involved in the administration of Anticholium® receive a copy of this letter and review the information.
Sincerely,
Frauke Weiß
CEO
Enclosure: Anticholium® Full Prescribing Information
|
US Product |
Import Product |
|
|
Product name |
Physostigmine Salicylate Injection |
ANTICHOLIUM® |
|
Disclaimer |
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. |
Disclaimer: Anticholium® (physostigmine salicylate) is not FDA approved, but is imported with foreign, non-U.S. labeling under a temporary importation license to address supply shortage. |
|
Dosage Form |
solution for injection |
solution for injection or infusion |
|
Label |
|
|
|
Composition |
Physostigmine Salicylate Injection is available in 2 mL ampules, each mL containing 1 mg of Physostigmine Salicylate in a vehicle composed of sodium metabisulfite 0.1%, benzyl alcohol 2.0% as a preservative in Water for Injection. |
Anticholium® is available in 5 mL ampules, each mL containing 0.4 mg physostigmine salicylate in a vehicle composed of sodium edetate (Ph. Eur.), nitrogene and water for injection. |
|
Indications |
INDICATIONS AND USAGE: To reverse the effect upon the central nervous system, caused by clinical or toxic dosages of drugs capable of producing the anticholinergic syndrome. |
Anticholium® is indicated for: 1. the use as an antidote and/or antagonist in case of intoxication with and/or overdose of:
2. the treatment of postoperative disorders:
|
|
Contraindications |
Physostigmine Salicylate Injection should not be used in the presence of asthma, gangrene, diabetes, cardiovascular disease, mechanical obstruction of the intestine or urogenital tract or any vagotonic state, and in patients receiving choline esters and depolarizing neuromuscular blocking agents (decamethonium, succinylcholine). For post-anesthesia, the concomitant use of atropine with physostigmine salicylate is not recommended, since the atropine antagonizes the action of physostigmine. |
Anticholium® should not be used in the presence of hypersensitivity to physostigmine salicylate, bronchial asthma, ulcers associated with tissue destruction (gangrene), coronary heart disease, constipation (mechanical obstipation), mechanical ischuria (urinary retention), hereditary form of muscle atrophy (myotonic dystrophy), closed traumatic brain injuries, intestinal obstruction, spasms in the urinary tract collection system, inactivation of nerve and muscle cells after administration of drugs to relax the muscles (depolarization block after depolarizing muscle relaxants), in case of intoxication due to “irreversibly acting” cholinesterase inhibitors (drugs used to treat dementia), intoxication with phosphoric acid esters or barbiturates. |
|
Precautions |
PRECAUTIONS: Because of the possibility of hypersensitivity in an occasional patient, atropine sulfate injection should always be at hand since it is an antagonist and antidote for physostigmine. |
Talk to your doctor before you are given Anticholium® if you suffer from diabetes mellitus, slowed heart rate (bradycardia), disturbances of the atrioventricular conduction system, Parkinson’s disease or ulcerative colitis. Hypersensitivity reactions may occur after administration of this medicine. These reactions may vary individually and may also cause life-threatening conditions. It is recommended to have emergency medication and medical aids ready. A risk/benefit analysis should be performed, and discontinuation of treatment should be considered. Acute cardiac arrest may be possible during treatment with tricyclic antidepressants; therefore, Anticholium® should only be considered as an antidote for this indication while the patient has continuous ECG monitoring. |
|
Interactions |
- |
Tell your doctor or pharmacist if you are taking/using, have recently taken/used or intend to take/use any other medicines. In case of simultaneous administration of other cholinesterase inhibitors (e.g. medicines for the treatment of dementia), caution should be exercised because of the potentiating effect. In intoxication with depolarizing muscle relaxants of the suxamethonium type, Anticholium® is contraindicated! |
|
Warnings |
Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people. If excessive symptoms of salivation, emesis, urination and defecation occur, the use of Physostigmine Salicylate Injection should be terminated. If excessive sweating or nausea occur, the dosage should be reduced. Intravenous administration should be at a slow, controlled rate, no more than 1 mg per minute (see dosage). Rapid administration can cause bradycardia, hypersalivation leading to a respiratory difficulties and possible convulsions. An overdosage of Physostigmine Salicylate Injection can cause a cholinergic crisis. |
Talk to your doctor before you are given Anticholium® if you suffer from diabetes mellitus, slowed heart rate (bradycardia), disturbances of the atrioventricular conduction system, Parkinson’s disease or ulcerative colitis. Hypersensitivity reactions may occur after administration of this medicine. These reactions may vary individually and may also cause life-threatening conditions. It is recommended to have emergency medication and medical aids ready. A risk/benefit analysis should be performed and discontinuation of treatment should be considered. Acute cardiac arrest may be possible during treatment with tricyclic antidepressants; therefore, Anticholium® should only be considered as an antidote for this indication while the patient has continuous ECG monitoring. |
|
Dosage and Administration |
Past Anesthesia Care: 0.5 to 1.0 mg intramuscularly or intravenously. INTRAVENOUS ADMINISTRATION SHOULD BE AT A SLOW CONTROLLED RATE OF NO MORE THAN 1 MG PER MINUTE. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained. PEDIATRIC DOSAGE: Recommended dosage is 0.02 mg/kg; intramuscularly or by slow intravenous injection, no more than 0.5 mg per minute. If the toxic effects persist, and there is no sign of cholinergic effects, the dosage may be repeated at 5 to 10 minute intervals until a therapeutic effect is obtained or a maximum of 2 mg dosage is attained. IN ALL CASES OF POISONING, THE USUAL SUPPORTIVE MEASURES SHOULD BE UNDERTAKEN. |
Use in adults: For the treatment of postoperative awakening disorders: Slowly inject physostigmine intravenously at a dose of 0.04 mg/kg bodyweight (approx. 1 mg/min), the maximum individual dose is 2 mg. In case of insufficient effect, give subsequent injections after 5 to 20 minutes at the earliest, after a positive evaluation of the effect of the first injection. In cases of intoxication: Adults: Slowly inject 0.04 mg/kg bodyweight (2 mg) of physostigmine salicylate intravenously and subsequently inject 1-4 mg every 20 minutes. Repeat the effective dose if the intoxication symptoms recur, also in the form of a continuous infusion if deemed useful. Use in children and adolescents: In cases of intoxication: Infants: Start with a low dose of 0.5 mg of physostigmine salicylate administered intravenously, repeat this dose every 5 minutes up to the overall dose of 2 mg, as long as the toxic, anticholinergic symptoms continue to persist, and no cholinergic symptoms occur. Method of administration Intravenously slowly or as brief infusion in 50 ml physiological saline solution over 10-15 minutes. A general criterion for adequate physostigmine dosing is the recognizable recovery of mental abilities and responsiveness (e.g. specifying name, address, date). |
|
Overdosage |
Can cause a cholinergic crisis. Appropriate antidote is atropine sulfate. |
Intravenous administration of atropine up to normalization of the symptoms. Normally, half the amount of the administered physostigmine salicylate is sufficient. In case of intoxication, measures to prevent absorption are to be started immediately (such as gastric lavage, administration of medicinal charcoal and laxatives). The following information is intended for healthcare professionals: Overdose emergency measures, symptoms, antidotes In cases of intoxication, measures to prevent absorption such as gastric lavage, administration of medicinal charcoal and laxatives are to be started immediately. An overdose of Anticholium® can cause bradycardia, hypersalivation, vomiting, and generalized tonic-clonic seizures. Patients should be closely monitored by ECG. |
|
Adverse Reactions |
Nausea, vomiting and salivation; can be offset by reducing dosage. Bradycardia and convulsions, if intravenous administration is too rapid. See DOSAGE AND ADMINISTRATION. |
Like all medicines, Anticholium® can cause side effects, although not everybody gets them.
|
|
Usage in pregnancy |
Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child. |
Pregnancy and breast-feeding Pregnancy: There is no experience with the use of Anticholium® in pregnant women. Physostigmine, the active substance contained in Anticholium®, passes into the placenta. Animal studies are insufficient with respect to effects on pregnancy, embryonal/foetal development, birth and postnatal development. The potential risk for humans is unknown. For this reason, Anticholium® may only be administered to pregnant women if deemed absolutely necessary by the attending doctor. Breast-feeding: There is no experience with the use of Anticholium® during breastfeeding. It is not known whether physostigmine, the active substance contained in Anticholium®, is excreted in human breast milk. For this reason, Anticholium® may only be used during breastfeeding if deemed absolutely necessary by the attending doctor. |
|
Storage Conditions |
STORAGE: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. |
Store this medicine protected from light in the outer packaging, not above 25°C. In undamaged container: 3 years The infusion solution should be used immediately after preparation. Keep this medicine out of the reach of children. Do not use this medicine after the expiry date which is stated on the carton/label after EXP. The expiry date refers to the last day of that month. |
|
How Supplied |
NDC 17478-510-02 2 mL Ampules packed 10 per box, 1 mg per mL. |
Anticholium® is a clear, colorless to slightly reddish solution in glass ampules. Pack containing 5 ampules with 5 ml. |
Related/similar drugs
physostigmine
Dosage Forms and Strengths
Anticholium is available in 5 mL ampules, each mL containing 0.4 mg physostigmine salicylate in a vehicle composed of sodium edetate (Ph. Eur.) and water for injection.
Indications and Usage for Anticholium Injection
ANTICHOLIUM® is indicated for:
1. the use as an antidote and/or antagonist in case of intoxication with and/or overdose of:
- alcohol
- tropane alkaloids (hyoscyamine, atropine, scopolamine, as e.g. in brugmansia, datura, atropa belladonna)
- amanita pantherina and amanita muscaria
- tricyclic antidepressants (amitriptyline, imipramine, trimipramine, clomipramine, doxepine)
- antiemetics/antihistamines (phenothiazine, thioridazine, chlorpromazine, promethazine, diphenhydramine, dimenhydrinate)
- neuroleptic drugs (especially butyrophenones)
- benzodiazepines
- tolterodine, oxybutynine
- amantadine, diphenhydramine
- baclofen
- 4-hydroxybutyric acid (GHB)
- inhalation anesthetics
- ketamine
- 3-quinuclidinyl benzilate
2. the treatment of postoperative disorders:
- Central anticholinergic syndrome (CAS)
- Delayed postoperative awakening
- Shivering
Contraindications
ANTICHOLIUM® should not be used in the presence of hypersensitivity to physostigmine salicylate, bronchial asthma, ulcers associated with tissue destruction (gangrene), coronary heart disease, constipation (mechanical obstipation), mechanical ischuria (urinary retention), hereditary form of muscle atrophy (myotonic dystrophy), closed traumatic brain injuries, intestinal obstruction, spasms in the urinary tract collection system, inactivation of nerve and muscle cells after administration of drugs to relax the muscles (depolarization block after depolarizing muscle relaxants), in case of intoxication due to “irreversibly acting” cholinesterase inhibitors (drugs used to treat dementia), intoxication with phosphoric acid esters or barbiturates.
Warnings and Precautions
Talk to your doctor before you are given ANTICHOLIUM® if you suffer from diabetes mellitus, slowed heart rate (bradycardia), disturbances of the atrioventricular conduction system, Parkinson’s disease or ulcerative colitis.
Hypersensitivity reactions may occur after administration of this medicine. These reactions may vary individually and may also cause life-threatening conditions. It is recommended to have emergency medication and medical aids ready. A risk/benefit analysis should be performed, and discontinuation of treatment should be considered.
Acute cardiac arrest may be possible during treatment with tricyclic antidepressants; therefore, Anticholium should only be considered as an antidote for this indication while the patient has continuous ECG monitoring.
Drug Interactions
Tell your doctor or pharmacist if you are taking/ using, have recently taken/used or intend to take/ use any other medicines.
In case of simultaneous administration of other cholinesterase inhibitors (e.g. medicines for the treatment of dementia), caution should be exercised because of the potentiating effect. In intoxication with depolarizing muscle relaxants of the suxamethonium type, ANTICHOLIUM® is contraindicated!
Anticholium Injection Dosage and Administration
Use in adults:
For the treatment of postoperative awakening disorders:
Slowly inject physostigmine intravenously at a dose of 0.04 mg/kg bodyweight (approx. 1 mg/min), the maximum individual dose is 2 mg.
In case of insufficient effect, give subsequent injections after 5 to 20 minutes at the earliest, after a positive evaluation of the effect of the first injection.
In cases of intoxication:
Adults: Slowly inject 0.04 mg/kg bodyweight (2 mg) of physostigmine salicylate intravenously and subsequently inject 1-4 mg every 20 minutes. Repeat the effective dose if the intoxication symptoms recur, also in the form of a continuous infusion if deemed useful.
Use in children and adolescents:
In cases of intoxication:
Infants: Start with a low dose of 0.5 mg of physostigmine salicylate administered intravenously, repeat this dose every 5 minutes up to the overall dose of 2 mg, as long as the toxic, anticholinergic symptoms continue to persist, and no cholinergic symptoms occur.
Overdosage
Intravenous administration of atropine up to normalization of the symptoms.
Normally, half the amount of the administered physostigmine salicylate is sufficient. In case of intoxication, measures to prevent absorption are to be started immediately (such as gastric lavage, administration of medicinal charcoal and laxatives).
The following information is intended for healthcare professionals:
Overdose emergency measures, symptoms, antidotes
In cases of intoxication, measures to prevent absorption such as gastric lavage, administration of medicinal charcoal and laxatives are to be started immediately.
An overdose of ANTICHOLIUM® can cause bradycardia, hypersalivation, vomiting, and generalized tonic-clonic seizures.
Patients should be closely monitored by ECG.
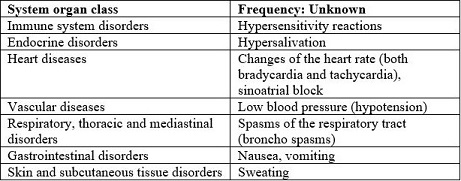
Adverse Reactions/Side Effects
Like all medicines, ANTICHOLIUM® can cause side effects, although not everybody gets them.
|
System organ class |
Frequency: Unknown |
|
Immune system disorders |
Hypersensitivity reactions |
|
Endocrine disorders |
Hypersalivation |
|
Heart diseases |
Changes of the heart rate (both bradycardia and tachycardia), sinoatrial block |
|
Vascular diseases |
Low blood pressure (hypotension) |
|
Respiratory, thoracic and mediastinal disorders |
Spasms of the respiratory tract (broncho spasms) |
|
Gastrointestinal disorders |
Nausea, vomiting |
|
Skin and subcutaneous tissue disorders |
Sweating |
Use In Specific Populations
Pregnancy and breast-feeding
Pregnancy:
There is no experience with the use of ANTICHOLIUM® in pregnant women.
Physostigmine, the active substance contained in ANTICHOLIUM®, passes into the placenta. Animal studies are insufficient with respect to effects on pregnancy, embryonal/foetal development, birth and postnatal development. The potential risk for humans is unknown.
For this reason, ANTICHOLIUM® may only be administered to pregnant women if deemed absolutely necessary by the attending doctor.
Breast-feeding:
There is no experience with the use of ANTICHOLIUM® during breastfeeding.
It is not known whether physostigmine, the active substance contained in ANTICHOLIUM®, is excreted in human breast milk. For this reason, ANTICHOLIUM® may only be used during breastfeeding if deemed absolutely necessary by the attending doctor.
Storage and Handling
Store this medicine protected from light in the outer packaging, not above 25°C.
In undamaged container: 3 years
The infusion solution should be used immediately after preparation.
Keep this medicine out of the reach of children.
Do not use this medicine after the expiry date which is stated on the carton/label after EXP. The expiry date refers to the last day of that month.
How is Anticholium Injection supplied
ANTICHOLIUM® is a clear, colorless to slightly reddish solution in glass ampules.
Pack containing 5 ampules with 5 ml.
PRINCIPAL DISPLAY PANEL - 5 x 5 mL Carton
ANTICHOLIUM®
2 mg/5 mL (0.4 mg/mL) solution for injection
Active substance: physostigmine salicylate
5 mL solution for injection contain:
2.0 mg physostigmine salicylate (0.4 mg/mL)
Sodium edetate (Ph. Eur.), sodium hydroxide for pH adjustment,
hydrochloric acid for pH adjustment, water for injections
DR. F. KÖHLER CHEMIE
Arzneimittel

| ANTICHOLIUM
physostigmine salicylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Provepharm Inc. (086861066) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr. Franz Köhler Chemie GmbH | 316512953 | manufacture(81284-831) | |
More about physostigmine
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: antidotes
- Breastfeeding
- En español