D-Cal Kids
Dosage form: granule
Ingredients: CALCIUM CARBONATE 750mg in 1g
Labeler: A&Z Pharmaceutical, Inc.
NDC code: 62211-239
Medically reviewed by Drugs.com. Last updated on Sep 25, 2023.
Calcium Carbonate 750 mg (in each pouch)
Antacid
Relieves:
- Heartburn
- Acid indigestion
- Sour stomach
- Upset stomach due to these symptoms
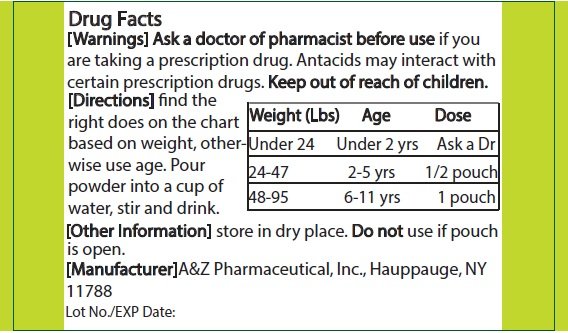
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product:
- do not take more than 1 pouch in a 24 hour period.
- use open pouch within two weeks.
- Find the right dose on chart below based on weight, otherwise use age.
- Pour powder into cup and add 15mg (0.51 oz.) of water, stir and drink.
Dosing Chart
|
Weight (lbs) |
Age |
Dose |
|
Under 24 |
Under 2 yrs |
Ask a doctor |
|
24-47 |
2-5 yrs |
1/2 pouch |
|
48-95 |
6-11 yrs |
1 pouch |
Cholecalciferol, dextrose, maltodextrin, sodium citrate
Store in a dry place.
Do not use if pouch is open or torn.
Mfg by: A&Z Pharmaceutical, Inc.
Hauppauge, NY 11788
Call toll free 1-800-480-1059 Monday through Friday 9AM-5PM
| D-CAL KIDS
calcium carbonate granule |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - A&Z Pharmaceutical, Inc. (080225262) |
| Registrant - A&Z Pharmaceutical, Inc. (080225262) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| A&Z Pharmaceutical, Inc. | 080225262 | manufacture(62211-239) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.