Humco Castor Oil
Dosage form: liquid
Ingredients: CASTOR OIL 1mg in 1mL
Labeler: Humco Holding Group, Inc.
NDC code: 0395-9113
Medically reviewed by Drugs.com. Last updated on Jun 12, 2023.
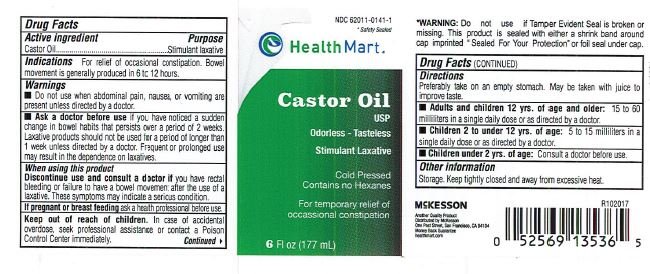
Drug Facts
Castor Oil
Stimulant Laxative
For the temporary relief of occasional constipation. Bowel movement is generally produced in 6 to 12 hours.
Do not use when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
if you have noticed a sudden change in bowel habits that persists over a period of 2 weeks. Laxative products should not be used for a period of longer than 1 week unless directed by a doctor. Frequent or prolonged use may result in the dependence on laxatives.
Discontinue use and consult a doctor if you have rectal bleeding or failure to have a bowel movement after the use of a laxative. These symptoms may indicate a serious condition.
ask a health professional before use
In case of accidental overdose, seek profssional assistance or contact a Poison Control Center immediately.
Preferably take on an empty stomach. May be taken with juice to improve taste.
Adults and children 12 yrs. of age and older: 1 to 4 tablesponfuls in a single daily dose or as directed by a doctor.
Children 2 to under 12 yrs. of age: 1 to 3 teaspoonfuls in a single dose or as directed by a doctor.
Children under 2 yrs. of age: Consult a doctor before use.
Do not use if Taper Evident Seal is broken or missing. This product is sealed with either a shrink band around cap imprinted "Sealed For your Protection" or foil seal under cap.
storage. Keep tightly closed and away from excessive heat.
None
| HUMCO CASTOR OIL
castor oil liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Humco Holding Group, Inc. (825672884) |
| Registrant - Humco Holding Group, Inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Humco Holding Group, Inc. | 825672884 | manufacture(0395-9113), analysis(0395-9113), pack(0395-9113), label(0395-9113) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.