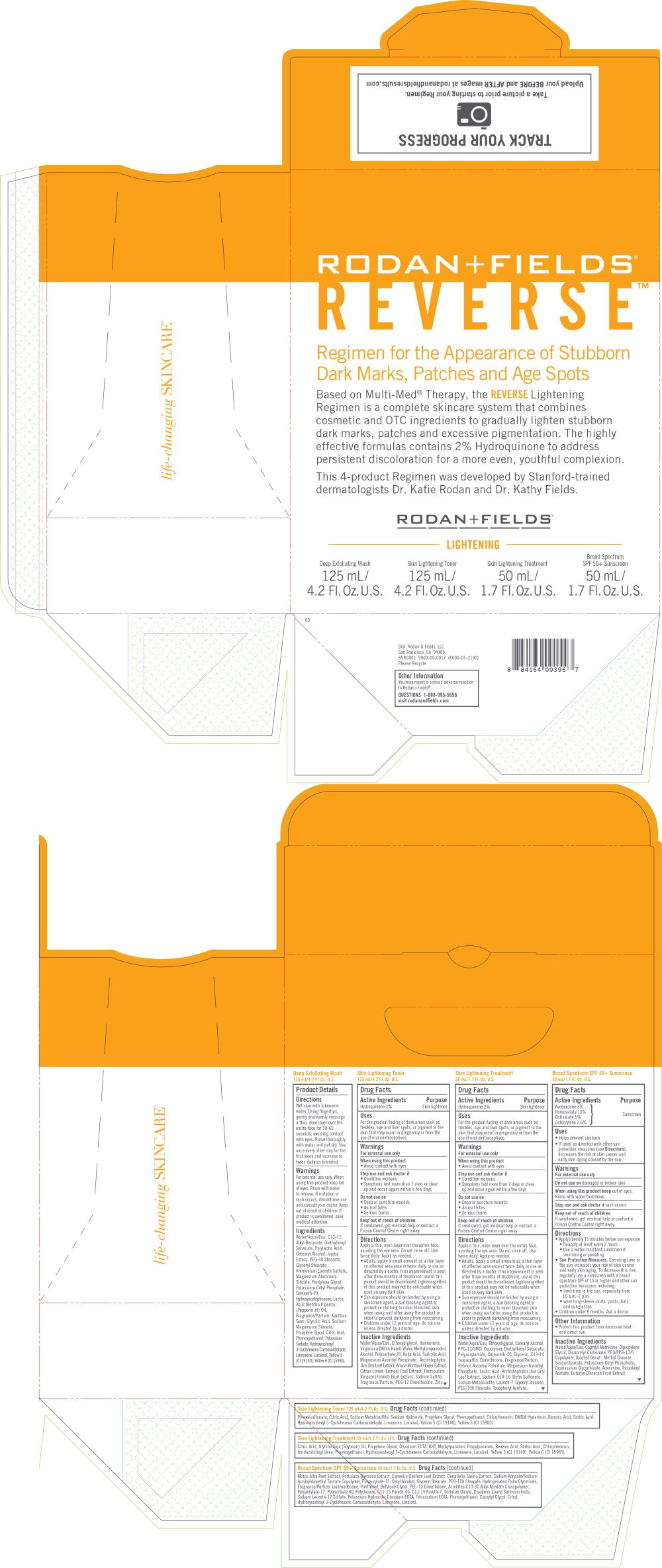
REVERSE Lightening Regimen for the Appearance of Stubborn Dark Marks, Patches and Age Spots
Dosage form: kit
Ingredients: Hydroquinone 2g in 100mL; Avobenzone 3.0g in 100mL, Homosalate 10g in 100mL, Octisalate 5g in 100mL, Octocrylene 2.6g in 100mL
Labeler: Rodan & Fields, LLC.
NDC code: 14222-5030
Medically reviewed by Drugs.com. Last updated on May 22, 2023.
Broad Spectrum SPF 50+ Sunscreen
50 mL/1.7 Fl. Oz. U.S.
Drug Facts
Avobenzone 3%
Homosalate 10%
Octisalate 5%
Octocrylene 2.6%
Sunscreen
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask doctor if rash occurs
If swallowed, get medical help or contact a Poison Control Center right away.
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- Children under 6 months: Ask a doctor
- Protect this product from excessive heat and direct sun.
Water/Aqua/Eau, Caprylyl Methicone, Dipropylene Glycol, Dicaprylyl Carbonate, PEG/PPG-17/6 Copolymer, Alcohol Denat., Methyl Glucose Sesquistearate, Potassium Cetyl Phosphate, Dipotassium Glycyrrhizate, Adenosine, Tocopheryl Acetate, Euterpe Oleracea Fruit Extract, Morus Alba Root Extract, Portulaca Oleracea Extract, Camellia Oleifera Leaf Extract, Dunaliella Salina Extract, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polyacrylate-15, Cetyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Hydrogenated Palm Glycerides, Fragrance/Parfum, Isohexadecane, Panthenol, Butylene Glycol, PEG-10 Dimethicone, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyacrylate-17, Polysorbate 80, Polydecene, C11-15 Pareth-40, C11-15 Pareth-7, Sorbitan Oleate, Disodium Lauryl Sulfosuccinate, Sodium Laureth-12 Sulfate, Potassium Hydroxide, Disodium EDTA, Tetrasodium EDTA, Phenoxyethanol, Caprylyl Glycol, Citral, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool.
Skin Lightening Treatment
50 mL/1.7 Fl. Oz. U.S.
Drug Facts
Hydroquinone 2%
Skin lightener
For the gradual fading of dark areas such as freckles, age and liver spots, or pigment in the skin that may occur in pregnancy or from the use of oral contraceptives.
For external use only
- Avoid contact with eyes
- Condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days
- Deep or puncture wounds
- Animal bites
- Serious burns
If swallowed, get medical help or contact a Poison Control Center right away.
Apply a thin, even layer over the entire face, avoiding the eye area. Do not rinse off. Use twice daily. Apply as needed.
- Adults: apply a small amount as a thin layer on affected area once or twice daily, or use as directed by a doctor. If no improvement is seen after three months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin.
- Sun exposure should be limited by using a sunscreen agent, a sun blocking agent,or protective clothing to cover bleached skin when using and after using the product in order to prevent darkening from reoccurring.
- Children under 12 years of age: do not use unless directed by a doctor.
Water/Aqua/Eau, Ethoxydiglycol, Cetearyl Alcohol, PPG-12/SMDI Copolymer, Diethylhexyl Sebacate, Polyacrylamide, Ceteareth-20, Glycerin, C13-14 Isoparaffin, Dimethicone, Fragrance/Parfum, Retinol, Ascorbyl Palmitate, Magnesium Ascorbyl Phosphate, Lactic Acid, Arctostaphylos Uva Ursi Leaf Extract, Sodium C14-16 Olefin Sulfonate, Sodium Metabisulfite, Laureth-7, Glyceryl Stearate, PEG-100 Stearate, Tocopheryl Acetate, Citric Acid, Glycine Soja (Soybean) Oil, Propylene Glycol, Disodium EDTA, BHT, Methylparaben, Propylparaben, Benzoic Acid, Sorbic Acid, Chlorphenesin, Imidazolidinyl Urea, Phenoxyethanol, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool, Yellow 5 (CI 19140), Yellow 6 (CI 15985).
Skin Lightening Toner
125 mL/4.2 Fl. Oz. U.S.
Drug Facts
Hydroquinone 2%
Skin lightener
For the gradual fading of dark areas such as freckles, age and liver spots, or pigment in the skin that may occur in pregnancy or from the use of oral contraceptives.
For external use only
- Avoid contact with eyes
- Condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days
- Deep or puncture wounds
- Animal bites
- Serious burns
If swallowed, get medical help or contact a Poison Control Center right away.
Apply a thin, even layer over the entire face, avoiding the eye area. Do not rinse off. Use twice daily. Apply as needed.
- Adults: apply a small amount as a thin layer on affected area once or twice daily, or use as directed by a doctor. If no improvement is seen after three months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin.
- Sun exposure should be limited by using a sunscreen agent, a sun blocking agent,or protective clothing to cover bleached skin when using and after using the product in order to prevent darkening from reoccurring.
- Children under 12 years of age: do not use unless directed by a doctor.
Water/Aqua/Eau, Ethoxydiglycol, Hamamelis Virginiana (Witch Hazel) Water, Methylpropanediol, Alcohol, Polysorbate 20, Kojic Acid, Salicylic Acid, Magnesium Ascorbyl Phosphate, Arctostaphylos Uva Ursi Leaf Extract, Arnica Montana Flower Extract, Citrus Limon (Lemon) Peel Extract, Foeniculum Vulgare (Fennel) Fruit Extract, Sodium Sulfite, Fragrance/Parfum, PEG-12 Dimethicone, Zinc Phenolsulfonate, Citric Acid, Sodium Metabisulfite, Sodium Hydroxide, Propylene Glycol, Phenoxyethanol, Chlorphenesin, DMDM Hydantoin, Benzoic Acid, Sorbic Acid, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool, Yellow 5 (CI 19140), Yellow 6 (CI 15985).
Deep Exfoliating Wash
125 mL/4.2 Fl. Oz. U.S.
Product Details
Wet skin with lukewarm water. Using fingertips, gently and evenly massage a thin, even layer over the entire face for 30-60 seconds, avoiding contact with eyes. Rinse thoroughly with water and pat dry. Use once every other day for the first week and increase to twice daily as tolerated.
For external use only. When using this product keep out of eyes. Rinse with water to remove. If irritation or rash occurs, discontinue use and consult your doctor. Keep out of reach of children. If product is swallowed, seek medical attention.
Water/Aqua/Eau, C12-15 Alkyl Benzoate, Diethylhexyl Sebacate, Polylactic Acid, Cetearyl Alcohol, Jojoba Esters, PEG-40 Stearate, Glyceryl Stearate, Ammonium Laureth Sulfate, Magnesium Aluminum Silicate, Pentylene Glycol, Potassium Cetyl Phosphate, Ceteareth-20, Hydroxyacetophenone, Lactic Acid, Mentha Piperita (Peppermint) Oil, Fragrance/Parfum, Xanthan Gum, Glycolic Acid, Sodium Magnesium Silicate, Propylene Glycol, Citric Acid, Phenoxyethanol, Potassium Sorbate, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limonene, Linalool, Yellow 5 (CI 19140), Yellow 6 (CI 15985).
RODAN + FIELDS®
REVERSE
2 Skin Lightening
Toner
RODAN + FIELDS®
REVERSE
3 Skin Lightening
Treatment
RODAN + FIELDS®
REVERSE
4 Broad Spectrum
SPF 50+ Sunscreen
RODAN + FIELDS®
REVERSE™
Regimen for the Appearance of Stubborn
Dark Marks, Patches and Age Spots
Based on Multi-Med® Therapy, the REVERSE Lightening
Regimen is a complete skincare system that combines
cosmetic and OTC ingredients to gradually lighten stubborn
dark marks, patches and excessive pigmentation. The highly
effective formulas contains 2% Hydroquinone to address
persistent discoloration for a more even, youthful complexion.
This 4-product Regimen was developed by Stanford-trained
dermatologists Dr. Katie Rodan and Dr. Kathy Fields.
RODAN + FIELDS®
LIGHTENING
Deep Exfoliating Wash
125 mL/
4.2 Fl.Oz. U.S.
Skin Lightening Toner
125 mL/
4.2 Fl.Oz. U.S.
Skin Lightening Treatment
50 mL/
1.7 Fl.Oz. U.S.
Broad Spectrum
SPF 50+ Sunscreen
50 mL/
1.7 Fl.Oz. U.S.
| REVERSE LIGHTENING REGIMEN FOR THE APPEARANCE OF STUBBORN DARK MARKS, PATCHES AND AGE SPOTS
hydroquinone, avobenzone, homosalate, octisalate, octocrylene kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Rodan & Fields, LLC. (051659584) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.