signature care itch relief
Dosage form: cream
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 2g in 100g, ZINC ACETATE 0.1g in 100g
Labeler: Safeway
NDC code: 21130-154
Medically reviewed by Drugs.com. Last updated on Dec 6, 2023.
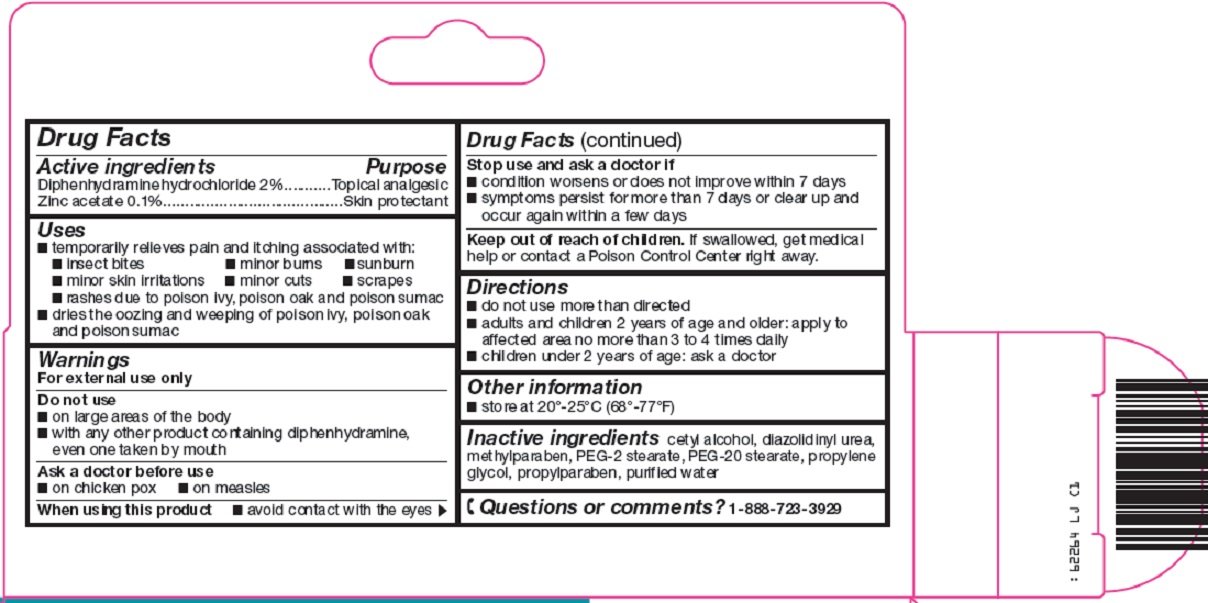
Active ingredients
Diphenhydramine hydrochloride 2%
Zinc acetate 0.1%
Purpose
Topical analgesic
Skin protectant
Uses
- •
- temporarily relieves pain and itching associated with:
- •
- insect bites
- •
- minor burns
- •
- sunburn
- •
- minor skin irritations
- •
- minor cuts
- •
- scrapes
- •
- rashes due to poison ivy, poison oak and poison sumac
- •
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
Warnings
For external use only
Do not use
- •
- on large areas of the body
- •
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- •
- on chicken pox
- •
- on measles
When using this product
- •
- avoid contact with the eyes
Stop use and ask a doctor if
- •
- condition worsens or does not improve within 7 days
- •
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- do not use more than directed
- •
- adults and children 2 years of age and older: apply to affected area no more than 3 to 4 times daily
- •
- children under 2 years of age: ask a doctor
Other information
- •
- store at 20°-25°C (68°-77°F)
Inactive ingredients
cetyl alcohol, diazolidinyl urea, methylparaben, PEG-2 stearate, PEG-20 stearate, propylene glycol, propylparaben, purified water
Questions or comments?
1-888-723-3929
| SIGNATURE CARE ITCH RELIEF
diphenhydramine hydrochloride, zinc acetate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Safeway (009137209) |
Document Id: 61232389-077d-46f0-b3fe-520c53dfbaa0
Set id: 3eedcd9f-8312-4d14-a0fe-6b00a00d3e73
Version: 2
Effective Time: 20191216
Safeway
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.