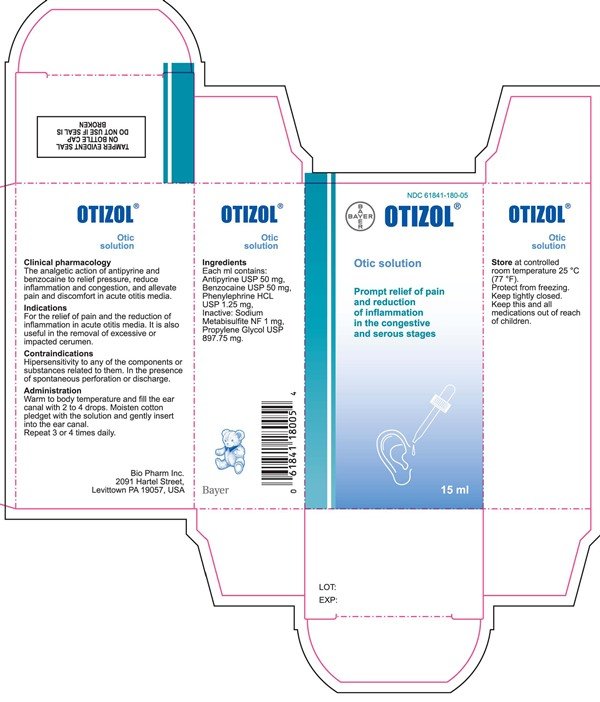
Ortizol Otic Solution
Dosage form: liquid
Ingredients: ANTIPYRINE 50mg in 1mL, BENZOCAINE 50mg in 1mL, PHENYLEPHRINE HYDROCHLORIDE 1.25mg in 1mL
Labeler: Bayer HealthCare LLC, Consumer Care
NDC code: 61841-180
Medically reviewed by Drugs.com. Last updated on Jan 26, 2024.
Antipyrine USP 50 mg
Benzocaine USP 50 mg
Phenylephrine HCL USP 1.25 mg
The analgetic action of antipyrine and
benzocaine to relief pressure. reduce
inflammation and congestion. and allevate
pain and discomfort in acute otitis media.
For the relief of pain and the reduction of
inflammation in acute otitis media. It is also
useful in the removal of excessive or
impacted cerumen.
Hipersensitivity to any of the components or
substances related to them. In the presence
of spontaneous perforation or discharge.
Warm to body temperature and fill the ear
canal with 2 to 4 drops. Moisten cotton
pledget with the solution and gently insert
into the ear canal.
Repeat 3 or 4 times daily.
Store at controlled
room temperature 25 °C
(77 ºF).
Protect from freezing.
Keep tightly closed.
Keep this and all
medications out of reach
of children.
Sodium
Metabisulfite NF 1 mg.
Propylene Glycol USP
897.75 mg.
| ORTIZOL
OTIC SOLUTION
antipyrine, benzocaine, phenylephrine hydrochloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bayer HealthCare LLC, Consumer Care (785159372) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.