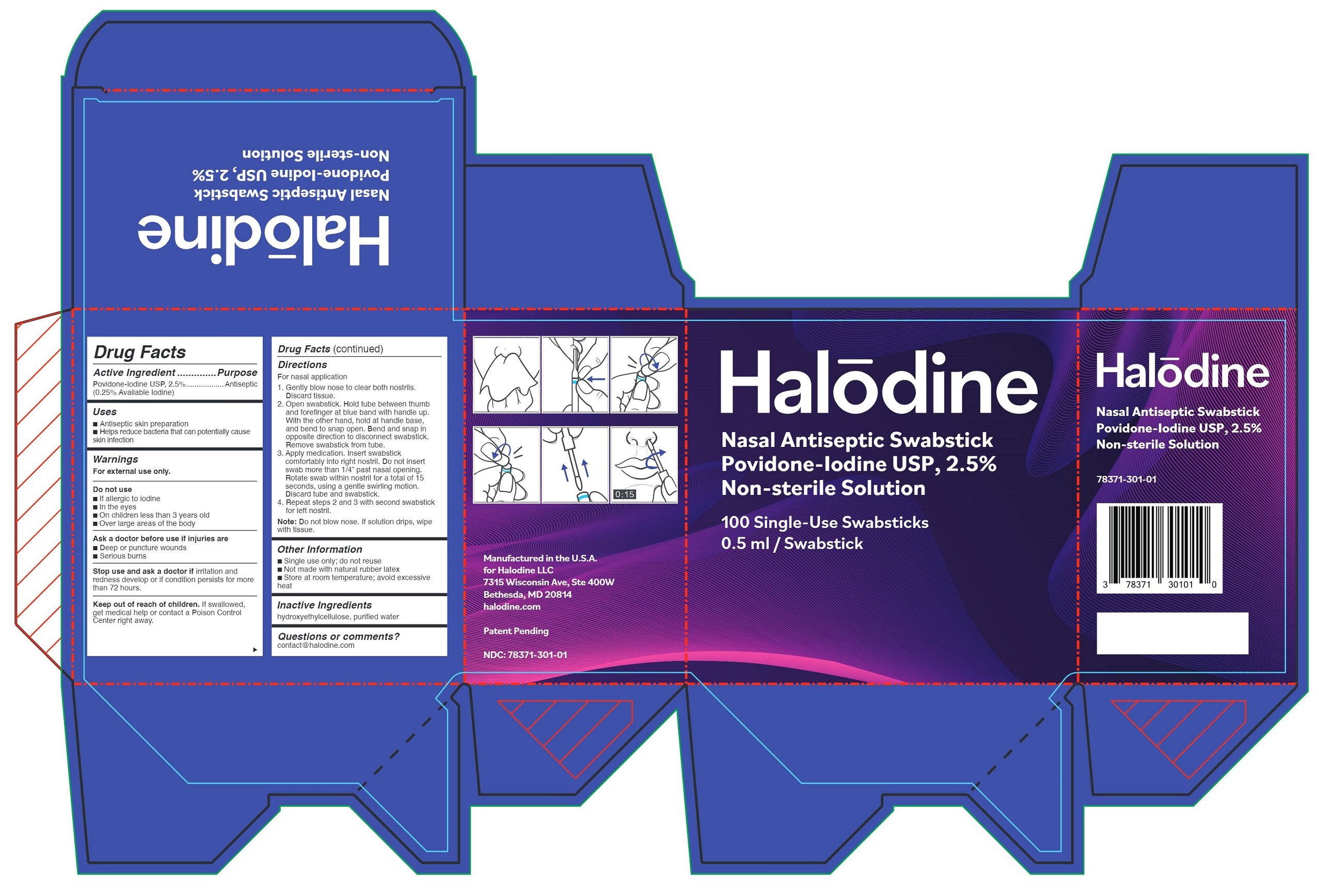
Halodine Nasal Antiseptic Swabstick
Dosage form: solution
Ingredients: POVIDONE-IODINE 2.5mg in 1mL
Labeler: Halodine LLC
NDC code: 78371-301
Medically reviewed by Drugs.com. Last updated on Aug 4, 2023.
Drug Facts
Active Ingredient
Povidone-Iodine USP, 2.5%
(0.25% Available Iodine)
Purpose
Antiseptic
Uses
- Antiseptic skin preparation
- Helps reduce bacteria that can potentially cause skin infection
Warnings
For external use only.
Do not use
- If allergic to iodine
- In the eyes
- On children less than 3 years old
- Over large areas of the body
Ask a doctor before use if injuries are
- Deep or puncture wounds
- Serious burns
Stop use and ask a doctor if
irritation and redness develop or if condition persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For nasal application
- Gently blow nose to clear both nostrils. Discard tissue.
- Open swabstick. Hold tube between thumb and forefinger at blue band with handle up. With other hand, hold handle at base, and bend to snap open. Bend and snap in opposite direction to disconnect swabstick. Remove swabstick from tube.
- Apply medication. Insert swabstick comfortably into right nostril. Do not insert swab more than 1/4” past nasal opening. Rotate swab within nostril for a total of 15 seconds, using a gentle swirling motion. Discard tube and swabstick.
- Repeat steps 2 and 3 with second swabstick for left nostril.
Note: Do not blow nose. If solution drips, wipe with tissue.
Other Information
- Single use only; do not reuse
- Not made with natural rubber latex
- Store at room temperature; avoid excessive heat
Inactive Ingredients
hydroxyethylcellulose, purified water
Questions or comments?
contact@halodine.com
| HALODINE NASAL ANTISEPTIC SWABSTICK
povidone-iodine solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Halodine LLC (117526113) |
Document Id: acd98be1-9473-188d-e053-2995a90a26e5
Set id: acd98be1-9472-188d-e053-2995a90a26e5
Version: 1
Effective Time: 20200814
Halodine LLC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.