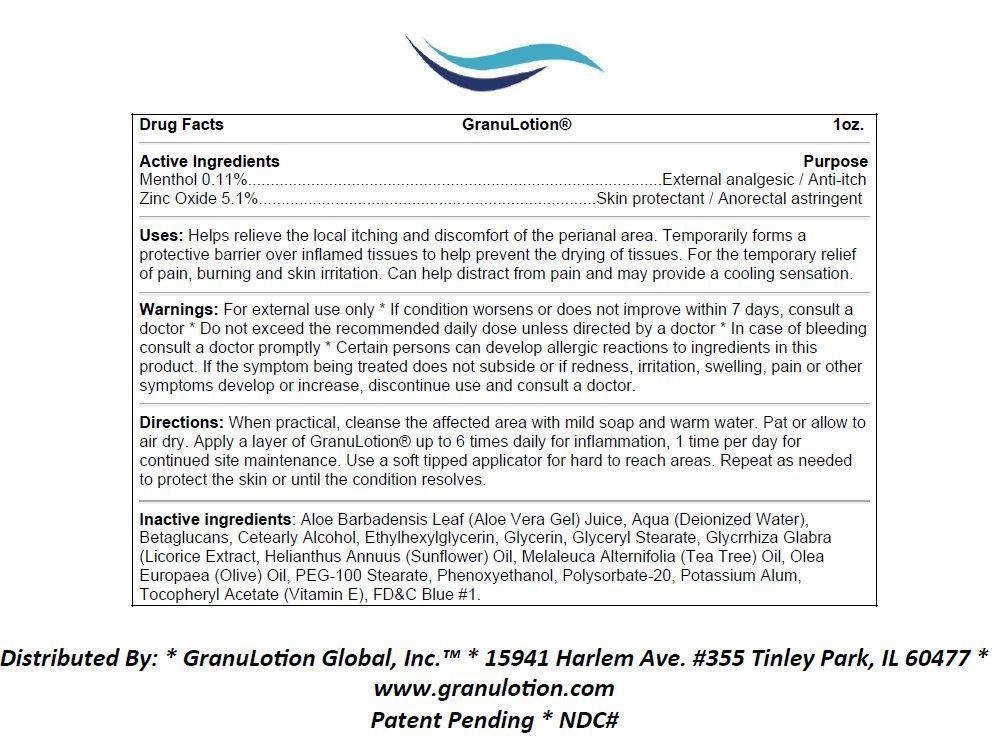
GranuLotion
Dosage form: lotion
Ingredients: MENTHOL 0.11g in 100g, ZINC OXIDE 5.1g in 100g
Labeler: Pure Source, LLC
NDC code: 65121-885
Medically reviewed by Drugs.com. Last updated on Jan 31, 2024.
Menthol 0.11%
Zinc Oxide 5.1%
External analgesic / Anti-itch
Skin protectant / Anorectal astringent
Helps relieve the local itching and discomfort of the perianal area. Temporarily forms a protective barrier over inflamed tissues to help prevent the drying of tissues. For the temporary relief of pain, burning and skin irritation. Can help distract from pain and may provide a cooling sensation.
For external use only * If condition worsens or does not improve within 7 days, consult a doctor * Do not exceed the recommended daily dose unless directed by a doctor * In case of bleeding consult a doctor promptly * Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase, discontinue use and consult a doctor.
When practical, cleanse the affected area with mild soap and warm water. Pat or allow to air dry. Apply a layer of GranuLotion® up to 6 times daily for inflammation, 1 time per day for continued site maintenance. Use a soft tipped applicator for hard to reach areas. Repeat as needed to protect the skin or until the condition resolves.
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Betaglucans, Cetearly Alcohol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Glycrrhiza Glabra (Licorice Extract, Helianthus Annuus (Sunflower) Oil, Melaleuca Alternifolia (Tea Tree) Oil, Olea Europaea (Olive) Oil, PEG-100 Stearate, Phenoxyethanol, Polysorbate-20, Potassium Alum, Tocopheryl Acetate (Vitamin E), FDandC Blue 1.
| GRANULOTION
menthol, zinc oxide lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Pure Source, LLC (080354456) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pure Source, LLC | 080354456 | manufacture(65121-885) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.