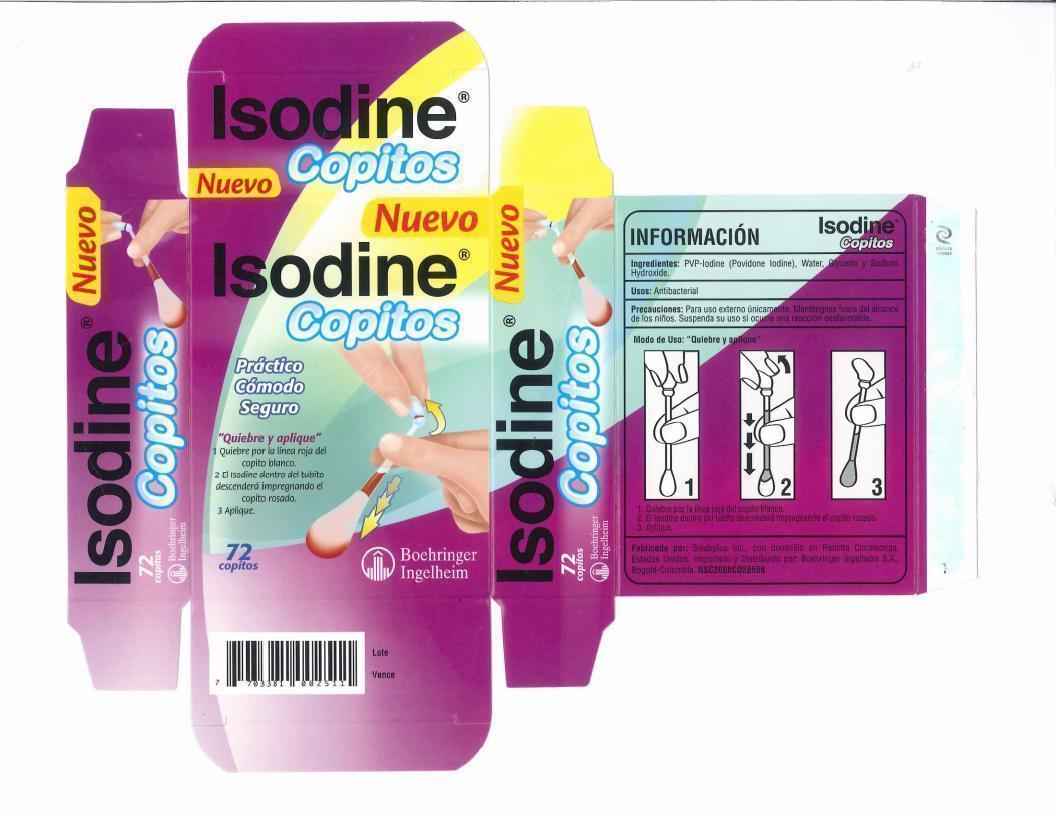
Isodine Copitos
Dosage form: solution
Ingredients: Povidone-Iodine 10mg in 1mL
Labeler: Swabplus Inc.
NDC code: 65734-511
Medically reviewed by Drugs.com. Last updated on Feb 6, 2024.
Active ingredient
Povidone iodine 10%, equal to 1% available Iodine
Antiseptic
Uses First aid to help prevent infection due to minor cuts, scrapes and burns.
if swallowed, get medical help of contact a Poison Control Center right away.
- Hold the swab vertically, with the color ring tip upwards, Hold n the center of the tube with one hand at the color ring with the other.
- Gently snap the tip with the color ring, Formula inside the tube flows down and fills the opposite tip.
- Apply the product to the affected area.
- Discard swab after use.
For external use only. Keep out of eyes and ear canal. Non-Sterile. Discontinue use if irritation.
Do not use. If you are sensitive to Iodine. Longer than one week unless directed by a doctor.
Stop use and ask a doctor. If you have deep or puncture wounds, or serious burns. redness, irritation, swelling or pain continue or increas., infedtion occurs.
For adults and children 2 and older: for minor wounds, burns and infections, apply directly to affected area. May be covered with a bandage.
Children under 2 years of age: Do not use, consult a doctor.
Other information: Store art room temperature, Avoid direct sunlight, excessive heat and moisture.
Glycerin, water
Patented and manufactured by Swabplus Inc. Rancho Cucamonga, CA 91730 USA Made in USA
Imported and Distributed by Boehringer Ingelheim
| ISODINE COPITOS
povidone-iodine solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Swabplus Inc. (876441549) |
| Registrant - Swabplus Inc. (876441549) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Swabplus Inc. | 876441549 | manufacture(65734-511), relabel(65734-511), repack(65734-511) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.