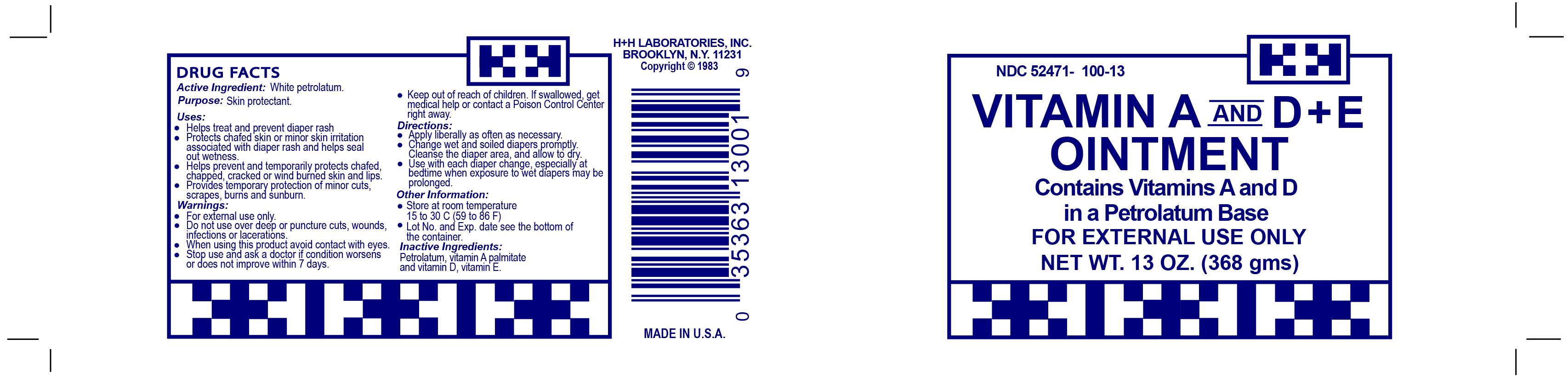
A and D plus E
Dosage form: ointment
Ingredients: PETROLATUM 937.5mg in 1g
Labeler: H&H Laboratories, Inc.
NDC code: 52471-100
Medically reviewed by Drugs.com. Last updated on May 4, 2023.
Active Ingredient (in each gram): White Petrolatum 93.5%
Purpose-Skin Protectant
Uses-Temporarily protects minor cuts, scrapes, and sunburn. Helps treat and prevent diaper rash. Protects chafed skin or minor skin irritation associated with diaper rash and helps seal out wetness. Temporarily protects and helps chapped or cracked skin and lips. Helps protect lips from drying effects of wind and cold weather.
Directions: Apply liberally as needed Change wet and soiled diapers promptly. Cleanse the diaper area and allow to dry. Use with each diaper change, especially at bedtime when exposure to wet diapers may be prolonged.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
For external use only. Do not use in eyes, on deep puncture wounds, animal bites, or serious burns or for more than 1 week unless directed by a doctor.
Stop use and ask a doctor if: A rash or allergic reaction develops. Condition worsens, persists, or recurs.
Inactive Ingredients: Corn Oil, Light Mineral Oil, Vitamin A Palmitate, Vitamin D, Vitamin E
| A AND D PLUS E
petrolatum ointment |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - H&H Laboratories, Inc. (151357175) |
| Registrant - H&H Laboratories, Inc. (151357175) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.