Assured Muscle Rub
Dosage form: gel
Ingredients: MENTHOL, UNSPECIFIED FORM 2.5g in 100g
Labeler: Greenbrier International, Inc.
NDC code: 33992-1136
Medically reviewed by Drugs.com. Last updated on Dec 18, 2023.
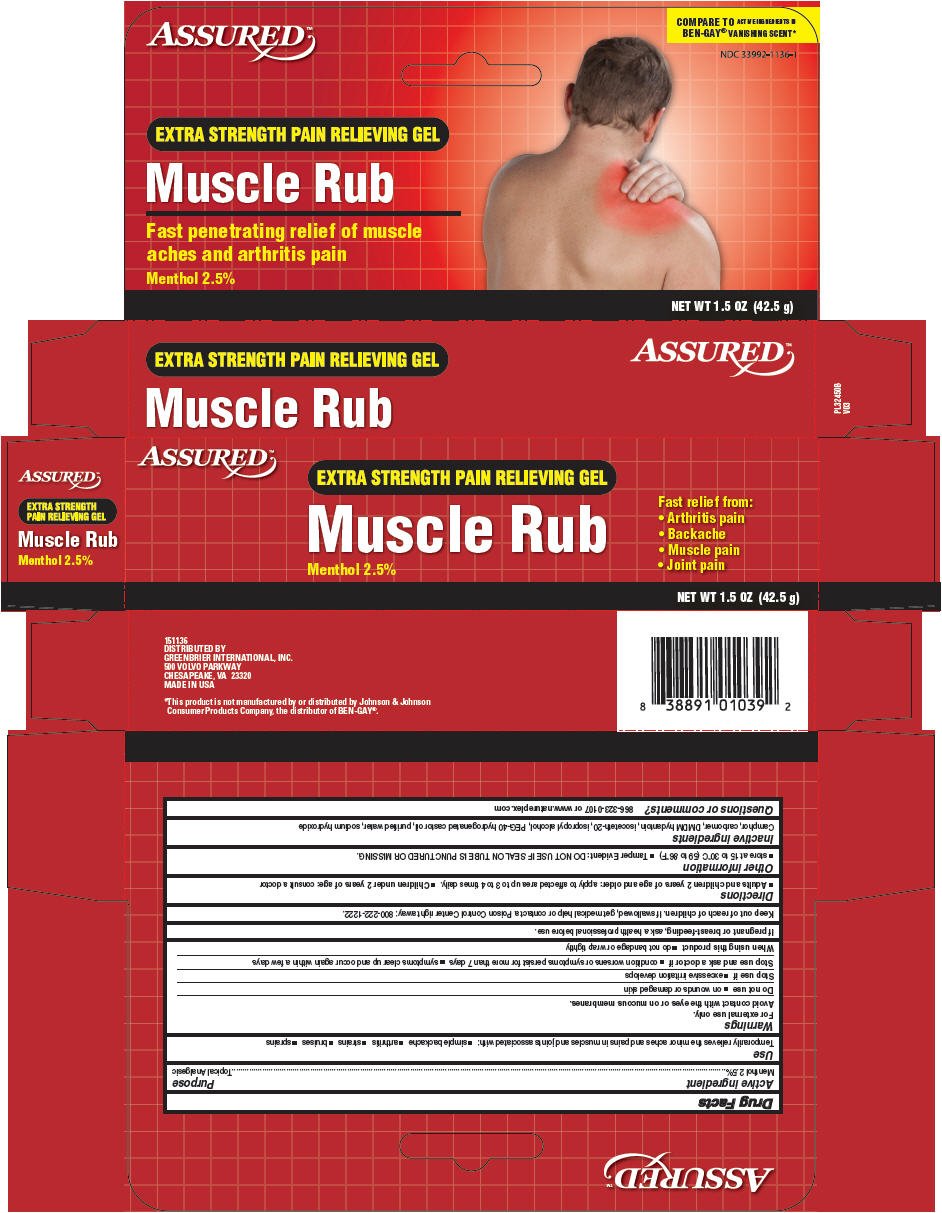
Drug Facts
Menthol 2.5%
Topical Analgesic
Temporarily relieves the minor aches and pains in muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
For external use only.
Avoid contact with the eyes or on mucous membranes.
- on wounds or damaged skin
- excessive irritation develops
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- do not bandage or wrap tightly
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away: 800-222-1222.
- Adults and children 2 years of age and older: apply to affected area up to 3 to 4 times daily.
- Children under 2 years of age: consult a doctor
- store at 15 to 30°C (59 to 86°F)
- Tamper Evident: DO NOT USE IF SEAL ON TUBE IS PUNCTURED OR MISSING.
Camphor, carbomer, DMDM hydantoin, isoceteth-20, isopropyl alcohol, PEG-40 hydrogenated castor oil, purified water, sodium hydroxide
866-323-0107 or www.natureplex.com
DISTRIBUTED BY
GREENBRIER INTERNATIONAL, INC.
500 VOLVO PARKWAY
CHESAPEAKE, VA 23320
| ASSURED
MUSCLE RUB
menthol gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Greenbrier International, Inc. (610322518) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Natureplex LLC | 062808196 | MANUFACTURE(33992-1136) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.