The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
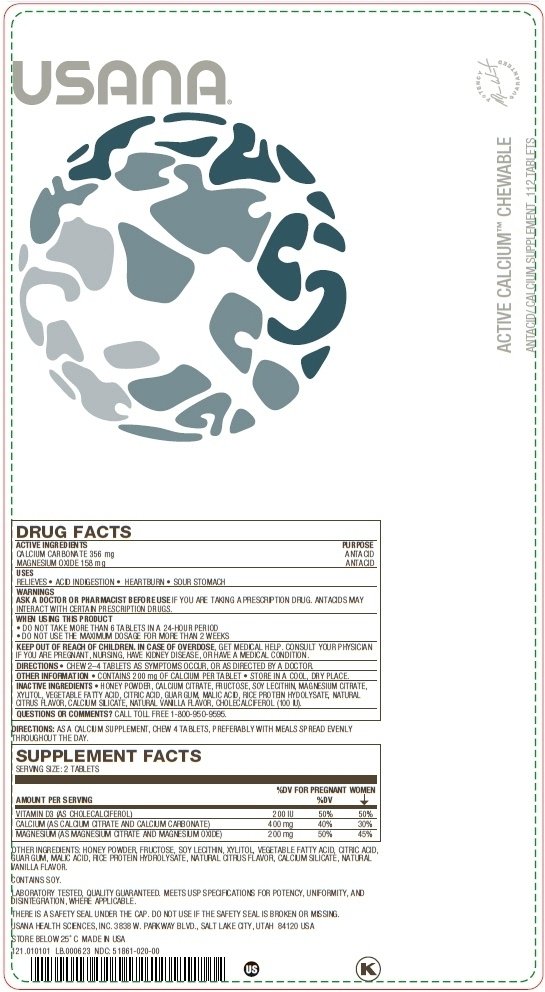
Usana Active Calcium
Dosage form: tablet, chewable
Ingredients: CALCIUM CARBONATE 356mg, MAGNESIUM OXIDE 158mg, CHOLECALCIFEROL 200[iU], CALCIUM CITRATE 77.9mg, MAGNESIUM CITRATE 44.4 mg
Labeler: Usana Health Sciences, Inc.
NDC code: 51861-020
DRUG FACTS
CALCIUM CARBONATE 356 mg
MAGNESIUM OXIDE 158 mg
ANTACID
RELIEVES
- ACID INDIGESTION
- HEARTBURN
- SOUR STOMACH
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE TAKING A PRESCRIPTION DRUG. ANTACIDS MAY INTERACT WITH CERTAIN PRESCRIPTION DRUGS.
- DO NOT TAKE MORE THAN 6 TABLETS IN A 24-HOUR PERIOD
- DO NOT USE THE MAXIMUM DOSAGE FOR MORE THAN 2 WEEKS
KEEP OUT OF REACH OF CHILDREN. IN CASE OF OVERDOSE, GET MEDICAL HELP.
CONSULT YOUR PHYSICIAN IF YOU ARE PREGNANT, NURSING, HAVE KIDNEY DISEASE, OR HAVE A MEDICAL CONDITION.
- CHEW 2-4 TABLETS AS SYMPTOMS OCCUR, OR AS DIRECTED BY A DOCTOR.
- CONTAINS 200 mg OF CALCIUM PER TABLET
- STORE IN A COOL, DRY PLACE.
- HONEY POWDER, CALCIUM CITRATE, FRUCTOSE, SOY LECITHIN, MAGNESIUM CITRATE, XYLITOL, VEGETABLE FATTY ACID, CITRIC ACID, GUAR GUM, MALIC ACID, RICE PROTEIN HYDOLYSATE, NATURAL CITRUS FLAVOR, CALCIUM SILICATE, NATURAL VANILLA FLAVOR, CHOLECALCIFEROL (100 IU).
CALL TOLL FREE 1-800-950-9595
AS A CALCIUM SUPPLEMENT, CHEW 4 TABLETS, PREFERABLY WITH MEALS SPREAD EVENLY THROUGHOUT THE DAY.
LABORATORY TESTED, QUALITY GUARANTEED. MEETS USP SPECIFICATIONS FOR POTENCY, UNIFORMITY, AND DISINTEGRATION, WHERE APPLICABLE.
THERE IS A SAFETY SEAL UNDER THE CAP. DO NOT USE IF SAFETY SEAL IS BROKEN OR MISSING.
USANA HEALTH SCIENCES, INC. 3838 W. PARKWAY BLVD., SALT LAKE CITY, UTAH 84120 USA
STORE BELOW 25˚C MADE IN USA
| USANA ACTIVE CALCIUM
calcium carbonate, magnesium oxide, cholecalciferol, calcium citrate, and magnesium citrate tablet, chewable |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Usana Health Sciences, Inc. (804413250) |
| Registrant - Usana Health Sciences, Inc. (804413250) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Usana Health Sciences, Inc. | 804413250 | manufacture(51861-020) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.