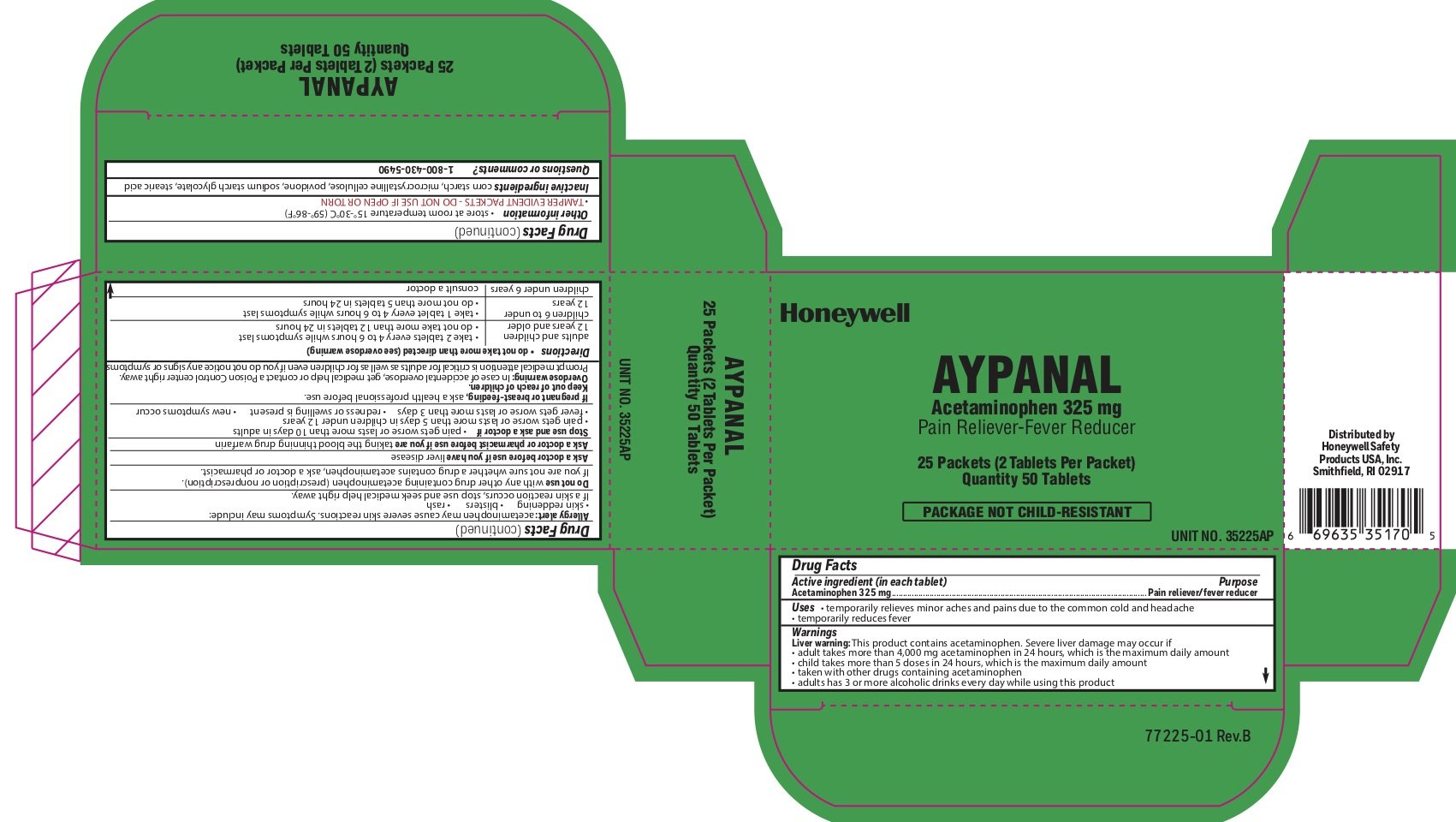
Aypanal
Dosage form: tablet
Ingredients: ACETAMINOPHEN 325mg
Labeler: Honeywell Safety Products USA, Inc
NDC code: 0498-2000
Medically reviewed by Drugs.com. Last updated on Jan 9, 2024.
Acetaminophen 325 mg
Pain reliever/fever reducer
- temporarily relieves minor aches and pains due to the common cold and headache
- temporarily reduces fever
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin rash occurs, stop use and seek medical help right away.
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
liver disease
you are taking the blood thinning drug warfarin
- pain gets worse or lasts more than 10 days in adults
- pain gets worse or lasts more than 5 days in children under 12 years
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning: In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- do not take more than directed (see overdose warning)
- adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
- children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- children under 6 years
- consult a doctor
- store at room temperature 15 o to 30 oC (59 o - 86 oF)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
1-800-430-5490
| AYPANAL
acetaminophen tablet |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| AYPANAL NON-ASPIRIN
acetaminophen tablet |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (079287321) |
| Registrant - Honeywell Safety Products USA, Inc (079287321) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Honeywell Safety Products USA, Inc | 079287321 | repack(0498-2001, 0498-2000) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.