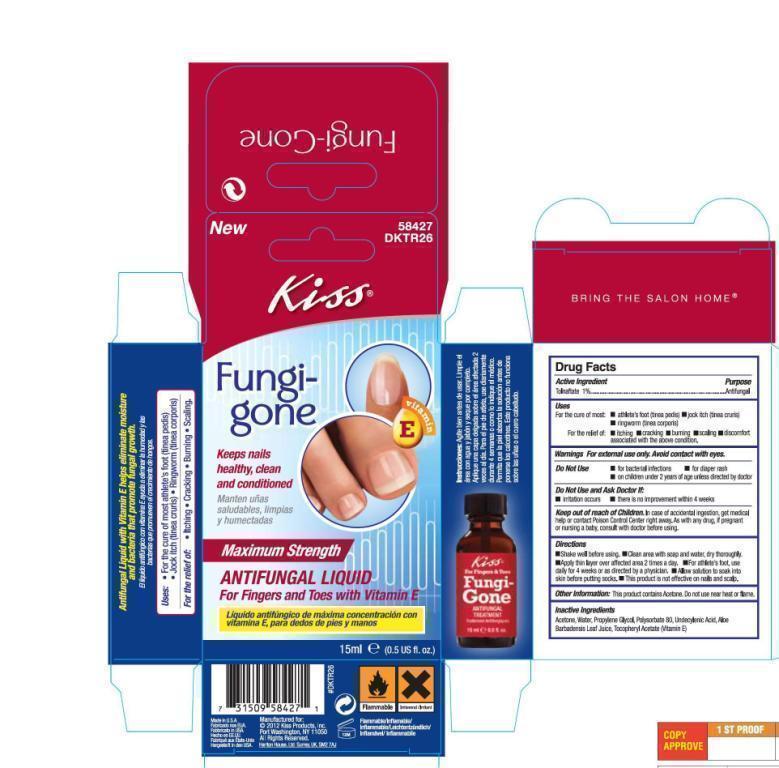
Kiss Fungi-Gone Anti-fungal Treatment
Dosage form: liquid
Ingredients: TOLNAFTATE 10mg in 1mL
Labeler: Kiss Products, Inc.
NDC code: 42432-101
Medically reviewed by Drugs.com. Last updated on Feb 15, 2024.
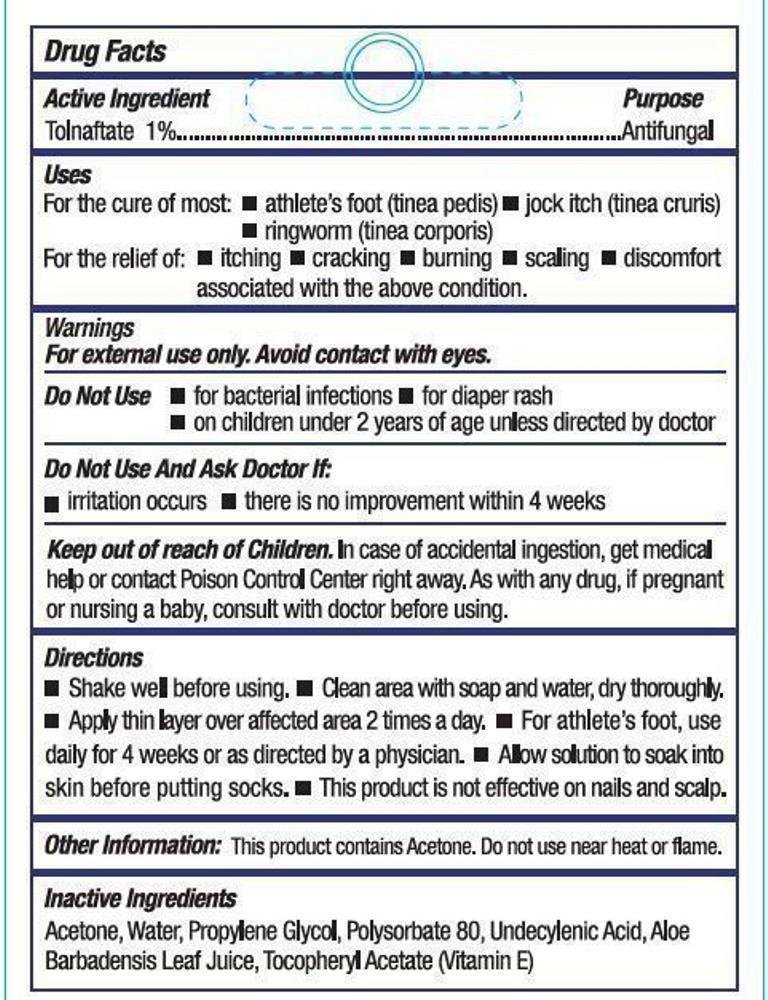
Tolnaftate 1%
Anti-fungal
For the cure of:
- athlete's foot (tinea pedis)
- jock itch (tinea cruris)
- ringworm (tinea corporis)
For the relief of:
- itching
- cracking
- burning
- scaling
- discomfort associated with the above condition
For external use only. Avoid contact with eyes.
- for bacterial infections
- for diaper rash
- on children under 2 years of age unless directed by a doctor
- irritation occurs
- there is no improvement within 4 weeks
In case of accidental ingestion, get medical help or contact Poison
Control Center right away. As with any drug, if pregnant or nursing a baby, consult with a doctor before using.
- Shake well before using
- Clean area with soap and water, dry thoroughly
- Apply thin layer over affected area 2 times a day
- For athlete's foot, use daily for 4 weeks or as directed by a physician
- Allow solution to soak into the skin before putting socks on
- This product is not effective on nails and scalp
This product contains Acetone. Do not use near heat or flame.
Acetone, Water, Propylene Glycol, Polysorbate 80, Undecylenic Acid, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate (Vitamin E)
| KISS FUNGI-GONE
ANTI-FUNGAL TREATMENT
tolnaftate liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Kiss Products, Inc. (626892020) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.