The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
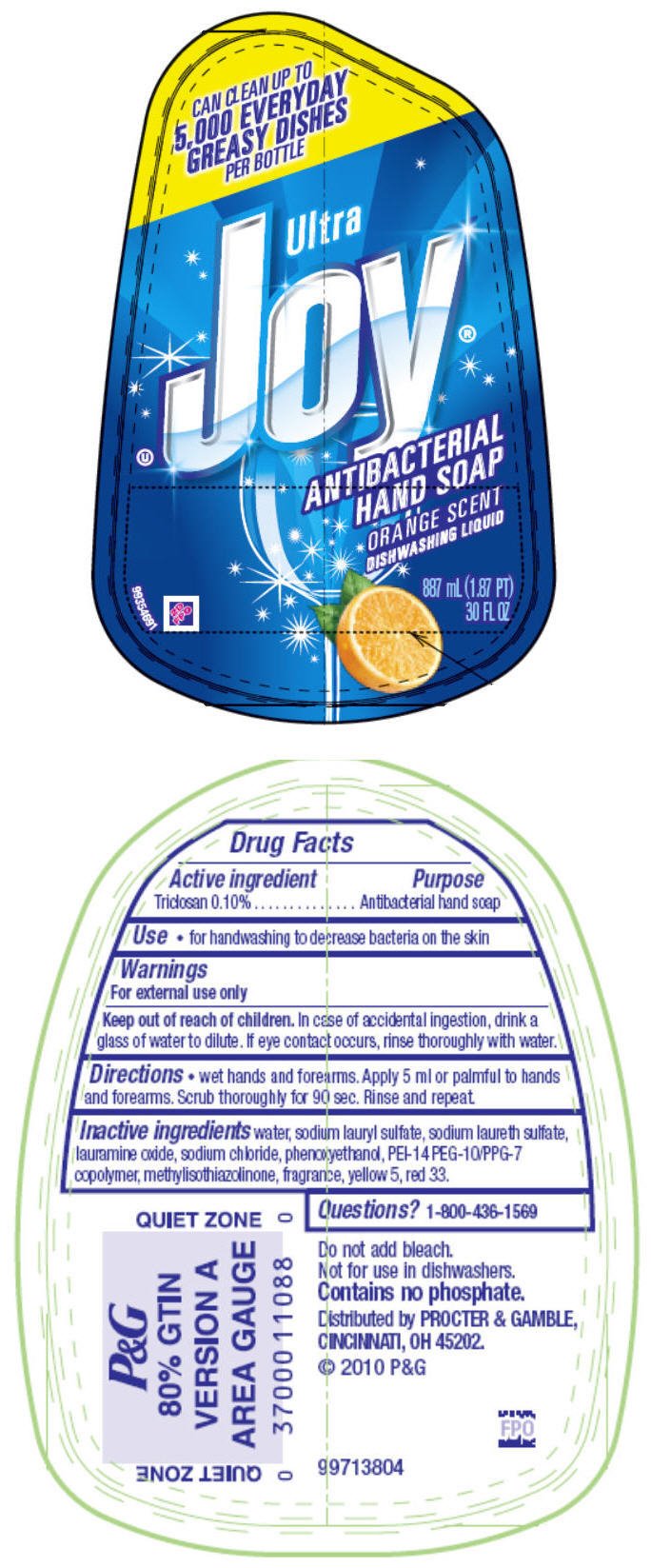
Joy Ultra Orange
Dosage form: soap
Ingredients: Triclosan 0.1g in 100mL
Labeler: Procter & Gamble Manufacturing Company
NDC code: 37000-607
DRUG FACTS
Triclosan 0.10%
Antibacterial hand soap
- For handwashing to decrease bacteria on the skin
For external use only
Keep out of reach of children.
In case of accidental ingestion, drink a glass of water to dilute. If eye contact occurs, rinse thoroughly with water.
- Wet hands and forearms. Apply 5 ml or palmful to hands and forearms. Scrub thoroughly for 90 sec. Rinse and repeat.
water, sodium lauryl sulfate, sodium laureth sulfate, lauramine oxide, sodium chloride, phenoxyethanol, PEI-14 PEG-10/PPG-7 copolymer, methylisothiazolinone, fragrance, yellow 5, red 33.
1-800-436-1569
Distributed by PROCTER & GAMBLE, CINCINNATI, OH 45202.
| JOY
ULTRA ORANGE
triclosan soap |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| The Procter & Gamble Manufacturing Company | 007130032 | ANALYSIS(37000-607), LABEL(37000-607), MANUFACTURE(37000-607), PACK(37000-607), RELABEL(37000-607), REPACK(37000-607) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.