The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
COMTREX Maximum Strength Cold and Cough Non-Drowsy
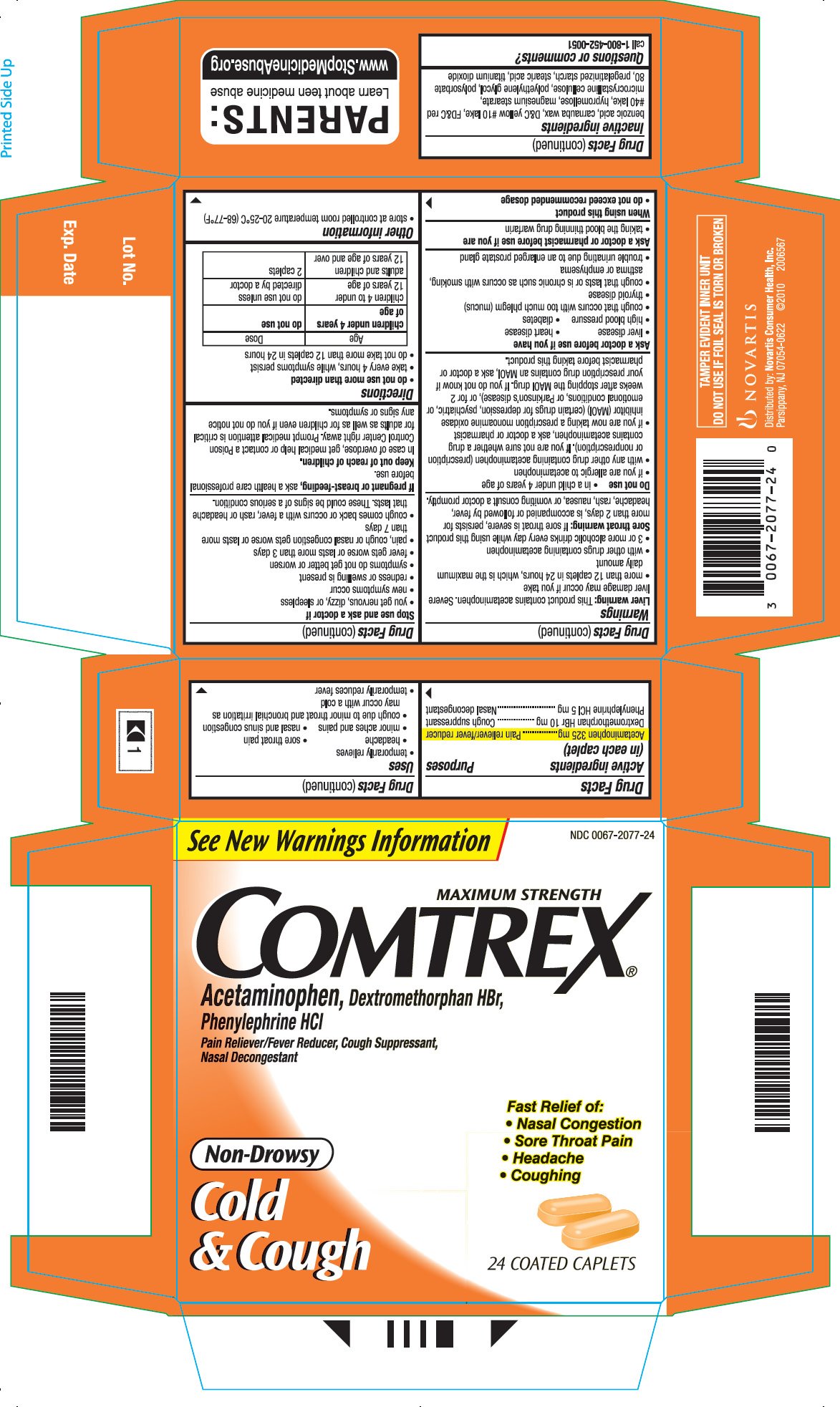
Dosage form: tablet, coated
Ingredients: ACETAMINOPHEN 325mg, DEXTROMETHORPHAN HYDROBROMIDE 10mg, PHENYLEPHRINE HYDROCHLORIDE 15mg
Labeler: Novartis Consumer Health, Inc.
NDC code: 0067-2077
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Phenylephrine HCl 5 mg
Pain reliever/fever reducer
Cough suppressant
Nasal decongestant
• temporarily relieve
• headache • sore throat pain
• minor aches and pains • nasal and sinus congestion
• cough due to minor throat and bronchial irritation as may occur with a cold
• temporarily reduces fever
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.
- in a child under 4 years of age
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
• liver disease • heart disease • high blood pressure • diabetes
• cough that occurs with too much phlegm (mucus) • thyroid disease
• cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
• trouble urinating due to an enlarged prostate gland
• taking the blood thinning drug warfarin
• do not exceed recommended dosage
• you get nervous, dizzy, or sleepless • new symptoms occur
• redness or swelling is present • symptoms do not get better or worsen
• fever gets worse or lasts more than 3 days
• pain, cough or nasal congestion gets worse or lasts more than 7 days
• cough comes back or occurs with a fever, rash or headache that lasts. These could be signs of a serious condition.
ask a health care professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
• do not use more than directed
• take every 4 hours, while symptoms persist
• do not take more than12 caplets in 24 hours
| Age | Dose |
| children under 4 years of age | do not use |
| children 4 to under 12 years of age | do not use unless directed by a doctor |
| adults and children 12 years of age and over | 2 caplets |
• store at controlled room temperature 20-25°C (68-77°F)
benzoic acid, carnauba wax, D&C yellow #10 lake, FD&C red #40 lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, stearic acid, titanium dioxide
call 1-800-452-0051
Distributed by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622
| COMTREX
MAXIMUM STRENGTH COLD AND COUGH NON-DROWSY
acetaminophen, dextromethorphan hbr, phenylephrine hcl tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Novartis Consumer Health, Inc. (879821635) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.