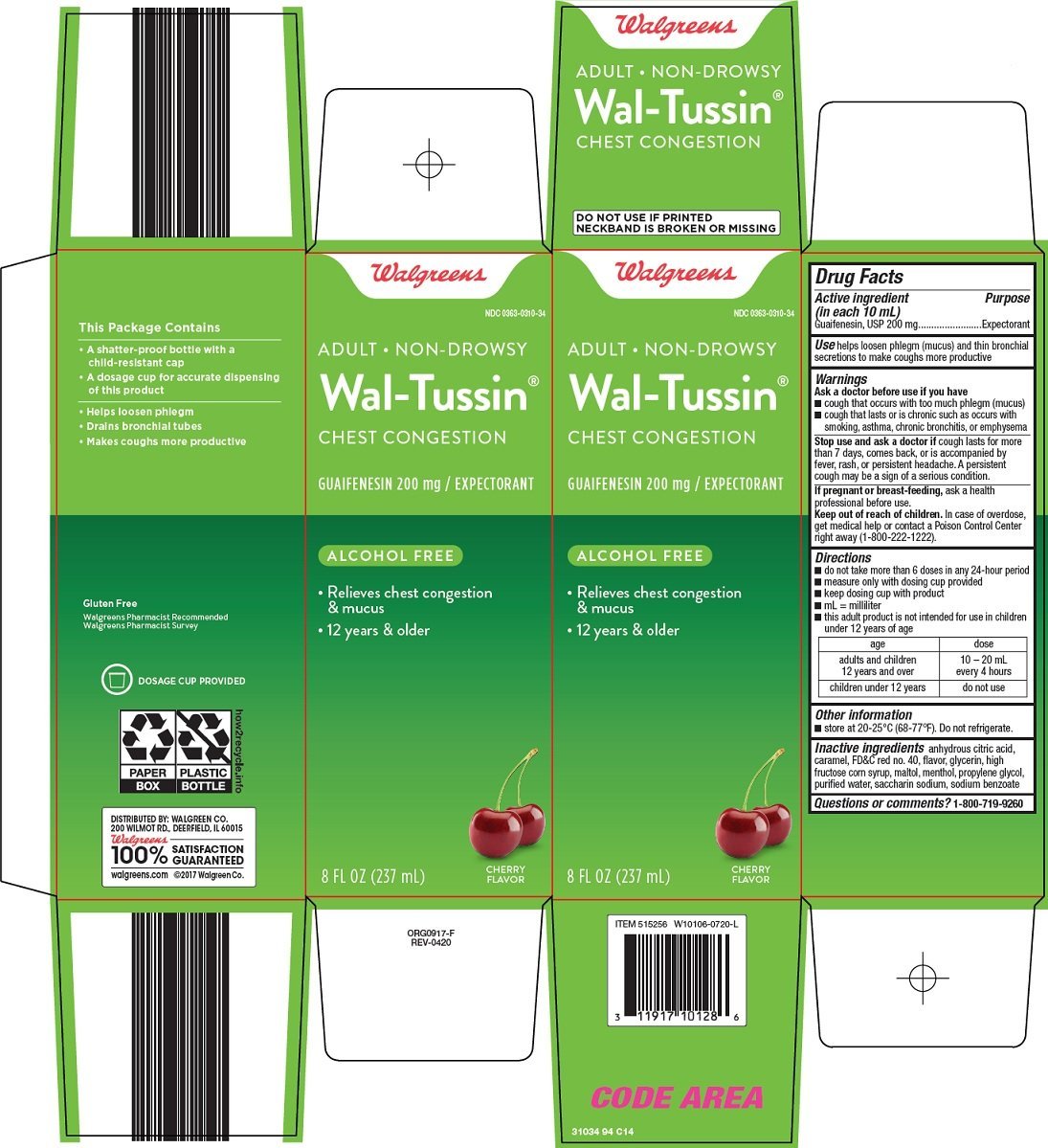
wal tussin adult chest congestion
Dosage form: solution
Ingredients: GUAIFENESIN 200mg in 10mL
Labeler: Walgreen Company
NDC code: 0363-0310
Medically reviewed by Drugs.com. Last updated on Jun 12, 2023.
Guaifenesin, USP 200 mg
Expectorant
helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
cough lasts for more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
10 – 20 mL every 4 hours |
|
children under 12 years |
do not use |
- •
- store at 20-25°C (68-77°F). Do not refrigerate.
anhydrous citric acid, caramel, FD&C red no. 40, flavor, glycerin, high fructose corn syrup, maltol, menthol, propylene glycol, purified water, saccharin sodium, sodium benzoate
1-800-719-9260
| WAL TUSSIN
ADULT CHEST CONGESTION
guaifenesin solution |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.