CLINIQUE ANTIPERSPIRANT DEODORANT ROLL-ON
Dosage form: liquid
Ingredients: ALUMINUM CHLOROHYDRATE 70mL in 100mL
Labeler: CLINIQUE LABORATORIES INC
NDC code: 49527-571
Medically reviewed by Drugs.com. Last updated on Apr 1, 2024.
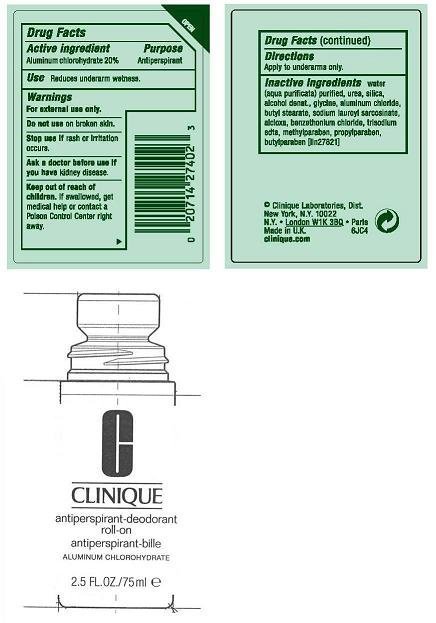
ACTIVE INGREDIENT:ALUMINUM CHLOROHYDRATE 20.00%
USES: DECREASES UNDERARM PERSPIRATION
WARNINGS:
- FOR EXTERNAL USE ONLY
- DO NOT USE ON BROKEN SKIN
- STOP USE IF RASH OR IRRITATION OCCURS
- ASK A DOCTOR BEFORE USE IF YOU HAVE KIDNEY DISEASE
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS: APPLY TO UNDERARMS ONLY.
inactive ingredients: water[] urea [] silica [] alcohol denat. [] glycine [] aluminum chloride [] butyl stearate [] sodium lauroyl sarcosinate [] alcloxa [] benzethonium chloride [] trisodium edta [] methylparaben [] propylparaben [] butylparaben iln27821
PRINCIPAL DISPLAY PANEL:
CLINIQUE
anti-perspirant deodorant roll-on
ALUMINUM CHLOROHYDRATE
2.5FL OZ./ 70ML
CLINIQUE LABORATORIES, DIST.
NEW YORK, NY 10022
6183
CLINIQUE.COM
CLINIQUE ANTIPERSPIRANT
DEODORANT ROLL-ON
aluminum chlorohydrate liquid |
|
|
|
|
|
|
|
|
|
|
CLINIQUE LABORATORIES INC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Medical Disclaimer

