The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
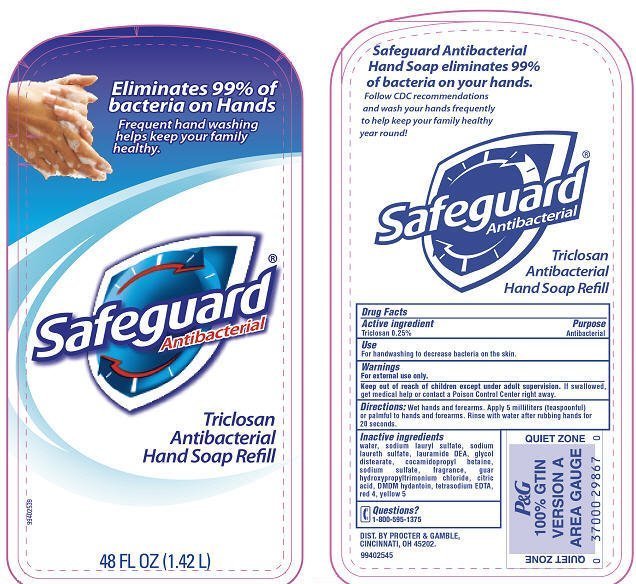
Safeguard
Dosage form: soap
Ingredients: Triclosan 0.25g in 100mL
Labeler: Procter & Gamble Manufacturing Company
NDC code: 37000-600
Drug Facts
Triclosan 0.25%
Antibacterial
For handwashing to decrease bacteria on the skin.
For external use only.
Keep out of reach of children except under adult supervision. If swallowed, get medical help or contact a Posion Control Center right away.
Wet hands and forearms. Apply 5 milliliters (teaspoonful) or palmful to hands and forearms. Rinse with water after rubbing hands for 20 seconds.
water, sodium lauryl sulfate, sodium laureth sulfate, lauramide DEA, glycol distearate, cocamidopropyl betaine, sodium sulfate, fragrance, guar hydroxypropyltrimonium chloride, citric acid, DMDM hydantoin, tetrasodium EDTA, red 4, yellow 5
1-800-595-1375
DIST. BY PROCTER & GAMBLE,
CINCINNATI, OH 45202.
| SAFEGUARD
triclosan soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Kleen Test Products | 168165814 | ANALYSIS(37000-600), MANUFACTURE(37000-600), RELABEL(37000-600), REPACK(37000-600) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.